

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50128540 5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-pyrimidine-2,4-diamine::CHEMBL75944
SMILES: Nc1ncc(CN2CCc3c(C2)[nH]c2ccccc32)c(N)n1
InChI Key: InChIKey=GQMRCYBXVKJXAP-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128540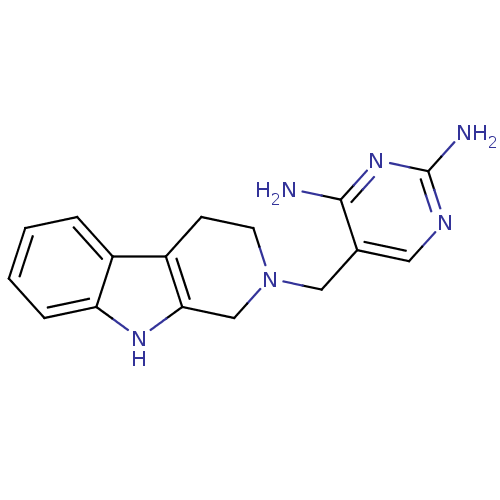 (5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity against TMP-Resistance Dihydrofolate reductase from Staphylococcus pneumoniae 1/1 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128540 (5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus (strain MW2)) | BDBM50128540 (5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-Resistance Dihydrofolate reductase from Staphylococcus aureus 157/4696 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128540 (5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||