

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50129555 (1R,2R)-2-((4E,6Z)-(2S,8R,9S,11R,13S,15S,16S)-7-cyano-8,16-dihydroxy-9,11,13,15-tetramethyl-18-oxo-oxacyclooctadeca-4,6-dien-2-yl)-cyclopentanecarboxylic acid::2-((1S,8R,9S,11R,13S,15S,18R)-7-Cyano-16-(S)-hydroxy-8-hydroxy-9,11,13,15-tetramethyl-18-oxo-oxacyclooctadeca-4,6-dien-2-yl)-cyclopentanecarboxylic acid::CHEMBL70590::cid_310900
SMILES: C[C@H]1C[C@@H](C)C[C@H](C)[C@@H](O)\C(=C/C=C/C[C@H](OC(=O)C[C@H](O)[C@@H](C)C1)[C@@H]1CCC[C@H]1C(O)=O)C#N
InChI Key: InChIKey=OJCKRNPLOZHAOU-UGKRXNSESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Threonyl-tRNA synthase (Phytophthora sojae) | BDBM50129555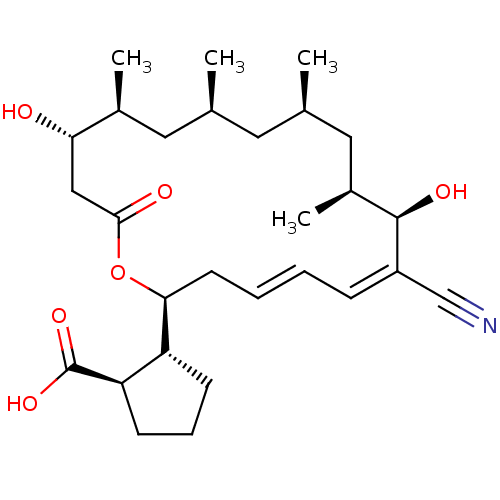 ((1R,2R)-2-((4E,6Z)-(2S,8R,9S,11R,13S,15S,16S)-7-cy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Agricultural University Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged Phytophthora sojae race 1 recombinant ThrRS expressed in Escherichia coli BL21 (Codonplus DE3) assessed as reduc... | J Agric Food Chem 60: 9874-81 (2012) Article DOI: 10.1021/jf302857x BindingDB Entry DOI: 10.7270/Q2B56NMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase RIPK2 (Homo sapiens (Human)) | BDBM50129555 ((1R,2R)-2-((4E,6Z)-(2S,8R,9S,11R,13S,15S,16S)-7-cy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >4.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2T15278 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-cysteine:1D-myo-inositol 2-amino-2-deoxy-alpha-D-glucopyranoside ligase (Mycobacterium tuberculosis) | BDBM50129555 ((1R,2R)-2-((4E,6Z)-(2S,8R,9S,11R,13S,15S,16S)-7-cy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant MBP-tagged MshC assessed as formation of fluorescently labeled Cys-GlcN-Ins by HPLC | Bioorg Med Chem Lett 21: 2480-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.042 BindingDB Entry DOI: 10.7270/Q26Q1XKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Threonyl-tRNA synthase (Phytophthora sojae) | BDBM50129555 ((1R,2R)-2-((4E,6Z)-(2S,8R,9S,11R,13S,15S,16S)-7-cy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Agricultural University Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged Phytophthora sojae race 1 recombinant ThrRS expressed in Escherichia coli BL21 (Codonplus DE3) assessed as reduc... | J Agric Food Chem 60: 9874-81 (2012) Article DOI: 10.1021/jf302857x BindingDB Entry DOI: 10.7270/Q2B56NMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta lactamase (Pseudomonas aeruginosa) | BDBM50129555 ((1R,2R)-2-((4E,6Z)-(2S,8R,9S,11R,13S,15S,16S)-7-cy...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >4.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2XS5T0R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division control protein 28 (Saccharomyces cerevisiae) | BDBM50129555 ((1R,2R)-2-((4E,6Z)-(2S,8R,9S,11R,13S,15S,16S)-7-cy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University Curated by ChEMBL | Assay Description Inhibition of cyclin-dependent kinase activity of Cdc28p/CDK2p in S. cerevisiae | Bioorg Med Chem Lett 13: 2235-7 (2003) BindingDB Entry DOI: 10.7270/Q20Z72N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||