

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50129640 3-(3-Methyl-but-2-enoylamino)-pyridine-2-carboxylic acid thiazol-2-ylamide::CHEMBL313629
SMILES: [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#7]-c1cccnc1-[#6](=O)-[#7]-c1nccs1
InChI Key: InChIKey=UJQGJJURAWVPHK-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine Aminopeptidase (MAP) (Escherichia coli (strain K12)) | BDBM50129640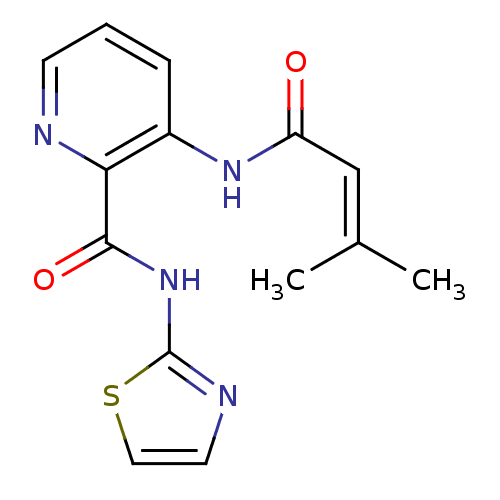 (3-(3-Methyl-but-2-enoylamino)-pyridine-2-carboxyli...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129640 (3-(3-Methyl-but-2-enoylamino)-pyridine-2-carboxyli...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||