

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50130699 1-(5-Hydroxymethyl-2,5-dihydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione; triphosphate::CHEMBL320969
SMILES: Cc1cn([C@@H]2O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)C=C2)c(=O)[nH]c1=O
InChI Key: InChIKey=ODSQODTUNULBHF-JGVFFNPUSA-J
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50130699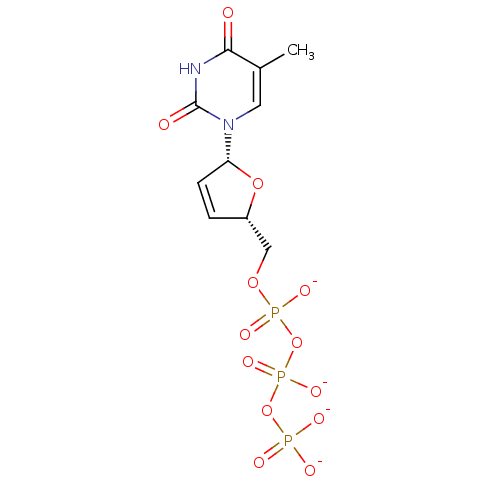 (1-(5-Hydroxymethyl-2,5-dihydro-furan-2-yl)-5-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibitory concentration required against Reverse transcriptase (RT) | J Med Chem 46: 3292-9 (2003) Article DOI: 10.1021/jm030116g BindingDB Entry DOI: 10.7270/Q2PR7WRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||