

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50131078 3-formylchromones::4-Oxo-4H-chromene-3-carbaldehyde::CHEMBL86905
SMILES: O=Cc1coc2ccccc2c1=O
InChI Key: InChIKey=FSMYWBQIMDSGQP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase NDM-1 (Klebsiella pneumoniae) | BDBM50131078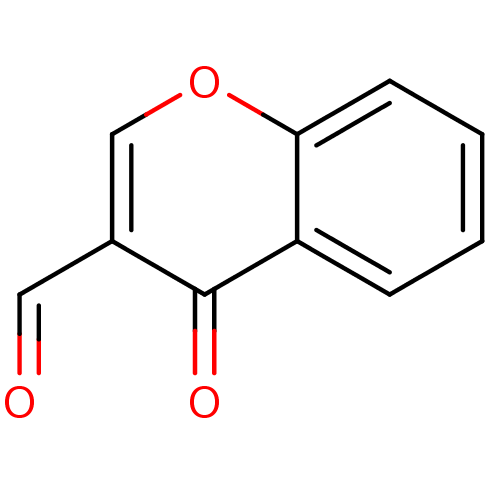 (3-formylchromones | 4-Oxo-4H-chromene-3-carbaldehy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Time-dependent inhibition of wild type Klebsiella pneumoniae 6xHis-tagged metallo-beta-lactamase NDM-1 expressed in Escherichia coli BL21 (DE3)pLysS | Bioorg Med Chem 24: 2947-2953 (2016) BindingDB Entry DOI: 10.7270/Q2XS5X91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase NDM-1 (Klebsiella pneumoniae) | BDBM50131078 (3-formylchromones | 4-Oxo-4H-chromene-3-carbaldehy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Time-dependent inhibition of wild type Klebsiella pneumoniae 6xHis-tagged metallo-beta-lactamase NDM-1 expressed in Escherichia coli BL21 (DE3)pLysS ... | Bioorg Med Chem 24: 2947-2953 (2016) BindingDB Entry DOI: 10.7270/Q2XS5X91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine Phosphorylase (TP) (Escherichia coli) | BDBM50131078 (3-formylchromones | 4-Oxo-4H-chromene-3-carbaldehy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Escherichia coli thymidine phosphorylase | Bioorg Med Chem 17: 2983-8 (2009) Article DOI: 10.1016/j.bmc.2009.03.020 BindingDB Entry DOI: 10.7270/Q2GX4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50131078 (3-formylchromones | 4-Oxo-4H-chromene-3-carbaldehy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B assessed as inhibition of kynuramine to 4-hydroxyquinoline conversion after 20 mins by fluorometry | Bioorg Med Chem Lett 22: 5480-4 (2012) Article DOI: 10.1016/j.bmcl.2012.07.025 BindingDB Entry DOI: 10.7270/Q2R212F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50131078 (3-formylchromones | 4-Oxo-4H-chromene-3-carbaldehy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A assessed as inhibition of kynuramine to 4-hydroxyquinoline conversion after 20 mins by fluorometry | Bioorg Med Chem Lett 22: 5480-4 (2012) Article DOI: 10.1016/j.bmcl.2012.07.025 BindingDB Entry DOI: 10.7270/Q2R212F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50131078 (3-formylchromones | 4-Oxo-4H-chromene-3-carbaldehy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B | Bioorg Med Chem Lett 13: 2561-3 (2003) BindingDB Entry DOI: 10.7270/Q2ZW1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||