 Found 7 hits for monomerid = 50131441
Found 7 hits for monomerid = 50131441 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50131441
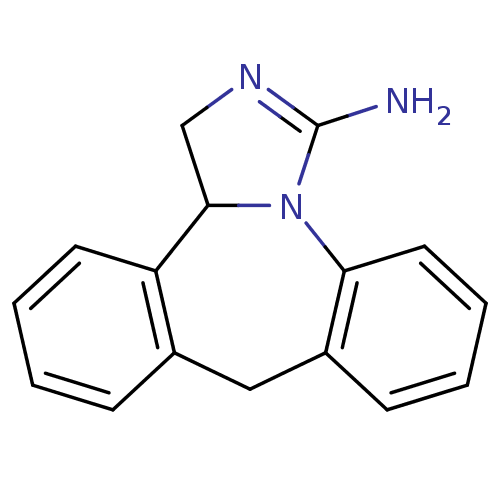
(9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-...)Show InChI InChI=1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Potassium channel HERG expressed in mammalian cells |
Bioorg Med Chem Lett 13: 2773-5 (2003)
BindingDB Entry DOI: 10.7270/Q2QZ2BGZ |
More data for this
Ligand-Target Pair | |
Multidrug and toxin extrusion protein 2
(Homo sapiens (Human)) | BDBM50131441

(9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-...)Show InChI InChI=1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human MATE2K-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay |
J Med Chem 56: 781-95 (2013)
Article DOI: 10.1021/jm301302s
BindingDB Entry DOI: 10.7270/Q2F76DWZ |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 2
(Homo sapiens (Human)) | BDBM50131441

(9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-...)Show InChI InChI=1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human OCT2-mediated ASP+ uptake expressed in HEK293 cells after 3 mins by fluorescence assay |
J Med Chem 56: 781-95 (2013)
Article DOI: 10.1021/jm301302s
BindingDB Entry DOI: 10.7270/Q2F76DWZ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50131441

(9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-...)Show InChI InChI=1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Potency against histamine H1 receptor on guinea pig ileum |
J Med Chem 38: 3351-60 (1995)
BindingDB Entry DOI: 10.7270/Q2ZW1N49 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50131441

(9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-...)Show InChI InChI=1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Reverse proteomics research institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against potassium channel HERG |
Bioorg Med Chem Lett 15: 2886-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.080
BindingDB Entry DOI: 10.7270/Q29S1S7C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50131441

(9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-...)Show InChI InChI=1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by whole cell patch clamp technique |
Bioorg Med Chem 16: 6252-60 (2008)
Article DOI: 10.1016/j.bmc.2008.04.028
BindingDB Entry DOI: 10.7270/Q25D8T25 |
More data for this
Ligand-Target Pair | |
Multidrug and toxin extrusion protein 1
(Homo sapiens (Human)) | BDBM50131441

(9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-...)Show InChI InChI=1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human MATE1-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay |
J Med Chem 56: 781-95 (2013)
Article DOI: 10.1021/jm301302s
BindingDB Entry DOI: 10.7270/Q2F76DWZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data