

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50132133 5-p-Tolyl-1H-pyrazole-3-carboxylic acid::CHEMBL341154::cid_818267
SMILES: Cc1ccc(cc1)-c1cc([nH]n1)C(O)=O
InChI Key: InChIKey=BGTWUOQARPOYER-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxycarboxylic acid receptor 2 (Rattus norvegicus) | BDBM50132133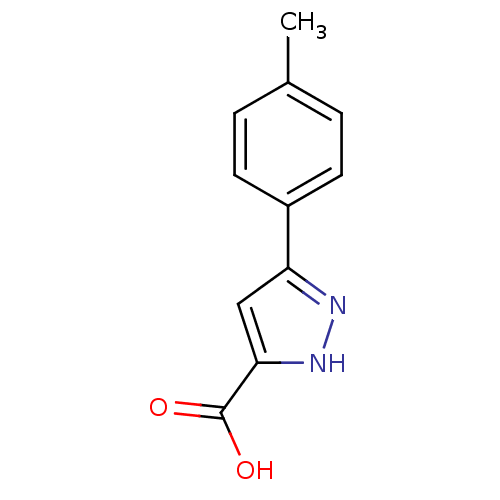 (5-p-Tolyl-1H-pyrazole-3-carboxylic acid | CHEMBL34...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research Curated by ChEMBL | Assay Description Inhibition of [3H]-nicotinic acid (20 nM) binding to nicotinic acid receptor in rat spleen membrane. | J Med Chem 46: 3945-51 (2003) Article DOI: 10.1021/jm030888c BindingDB Entry DOI: 10.7270/Q2V988TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50132133 (5-p-Tolyl-1H-pyrazole-3-carboxylic acid | CHEMBL34...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 expressed in Escherichia coli preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem 23: 4649-59 (2015) Article DOI: 10.1016/j.bmc.2015.05.052 BindingDB Entry DOI: 10.7270/Q2X068SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50132133 (5-p-Tolyl-1H-pyrazole-3-carboxylic acid | CHEMBL34...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 expressed in Escherichia coli preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem 23: 4649-59 (2015) Article DOI: 10.1016/j.bmc.2015.05.052 BindingDB Entry DOI: 10.7270/Q2X068SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50132133 (5-p-Tolyl-1H-pyrazole-3-carboxylic acid | CHEMBL34...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 expressed in Escherichia coli preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem 23: 4649-59 (2015) Article DOI: 10.1016/j.bmc.2015.05.052 BindingDB Entry DOI: 10.7270/Q2X068SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50132133 (5-p-Tolyl-1H-pyrazole-3-carboxylic acid | CHEMBL34...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 expressed in Escherichia coli preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem 23: 4649-59 (2015) Article DOI: 10.1016/j.bmc.2015.05.052 BindingDB Entry DOI: 10.7270/Q2X068SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50132133 (5-p-Tolyl-1H-pyrazole-3-carboxylic acid | CHEMBL34...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 8.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screen... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2CR5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||