

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50132298 1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-(trifluoromethyl)pyrimidine-2,4(1H,3H)-dione::1-((2R,5R)-4-Hydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-trifluoromethyl-1H-pyrimidine-2,4-dione::5-trifluoromethyl-2'-deoxyuridine::CHEMBL1129::TRIFLURIDINE::Trifluoromethyl Deoxyuridine::Viroptic::cid_6256::cid_6708818
SMILES: OC[C@H]1O[C@H](C[C@@H]1O)n1cc(c(=O)[nH]c1=O)C(F)(F)F
InChI Key: InChIKey=VSQQQLOSPVPRAZ-RRKCRQDMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50132298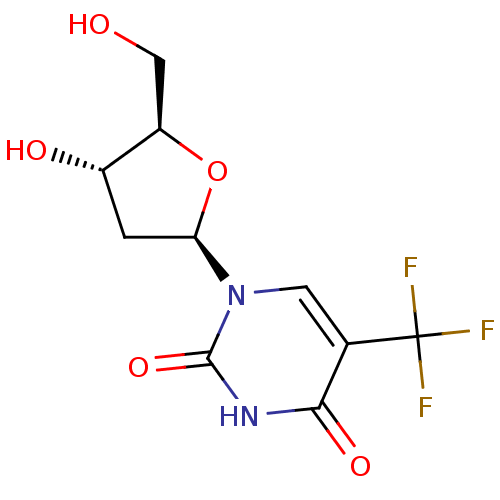 (1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibitory activity against thymidine monophosphate kinase (TMPK) in Mycobacterium tuberculosis | Bioorg Med Chem Lett 13: 3045-8 (2003) BindingDB Entry DOI: 10.7270/Q237798X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| exonuclease V (RecBCD complex), alpha chain (Escherichia coli str. K-12 substr. MG1655) | BDBM50132298 (1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2TB15CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50132298 (1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-... | Drug Metab Dispos 40: 2332-41 (2012) Article DOI: 10.1124/dmd.112.047068 BindingDB Entry DOI: 10.7270/Q2ZP488M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Macacine herpesvirus 1) | BDBM50132298 (1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahyd...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Inhibition of Herpes B virus recombinant thymidine kinase-mediated [3H]TdR phosphorylation | Antimicrob Agents Chemother 51: 2028-34 (2007) Article DOI: 10.1128/AAC.01284-06 BindingDB Entry DOI: 10.7270/Q2RN38R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| exonuclease V (RecBCD complex), alpha chain (Escherichia coli str. K-12 substr. MG1655) | BDBM50132298 (1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2TB15CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| exonuclease V (RecBCD complex), alpha chain (Escherichia coli str. K-12 substr. MG1655) | BDBM50132298 (1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 6.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | PubChem Bioassay (2013) BindingDB Entry DOI: 10.7270/Q21G0JXG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||