

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50132441 3-(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)-2-oxoindoline-5-sulfonamido)propanamide::3-{3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-ylidene]-2-oxo-2,3-dihydro-1H-indole-5-sulfonylamino}-propionamide::CHEMBL105427
SMILES: COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(=O)(=O)NCCC(N)=O)c2c1
InChI Key: InChIKey=FMAJMCVSAGAPDM-NVMNQCDNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50132441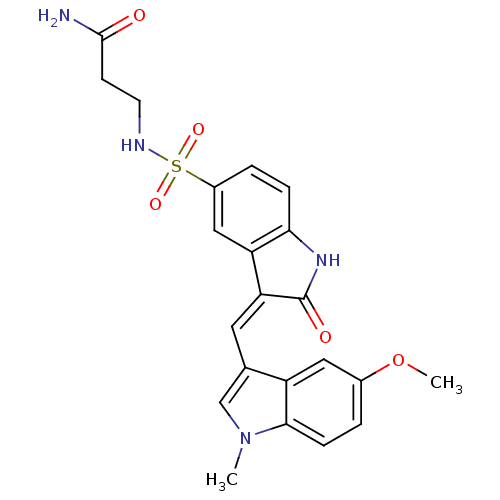 (3-(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of recombinant Syk (unknown origin) | Bioorg Med Chem Lett 19: 1944-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.049 BindingDB Entry DOI: 10.7270/Q2WM1D98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50132441 (3-(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis | Bioorg Med Chem Lett 13: 3111-4 (2003) BindingDB Entry DOI: 10.7270/Q2ZG6SS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Immunoglobulin epsilon Fc receptor (Homo sapiens (Human)) | BDBM50132441 (3-(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Effect on IgE/Fcepsilon RI triggered rat basophil cell (RBL-2H3) degranulation assessed by measuring the amount of 5-HT release | Bioorg Med Chem Lett 13: 3111-4 (2003) BindingDB Entry DOI: 10.7270/Q2ZG6SS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||