

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50133980 (2S)-2-{[(3aS,6S,6aR)-4-(Cyclopropylcarbonyl)-6-meth-yl-5-oxohexahydropyrrolo[3,2-b]pyrrol-1(2H)-yl]carbonyl}-N-(4-isopropoxyphenyl)pyrrolidine-1-carboxamide::(S)-2-((3aS,6S,6aR)-4-Cyclopropanecarbonyl-6-methyl-5-oxo-hexahydro-pyrrolo[3,2-b]pyrrole-1-carbonyl)-pyrrolidine-1-carboxylic acid (4-isopropoxy-phenyl)-amide::CHEMBL136258
SMILES: CC(C)Oc1ccc(NC(=O)N2CCC[C@H]2C(=O)N2CC[C@H]3[C@H]2[C@H](C)C(=O)N3C(=O)C2CC2)cc1
InChI Key: InChIKey=GBHJBOUYQXDQJT-IRNNPGBSSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human herpes virus 5 capsid protein P40 (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133980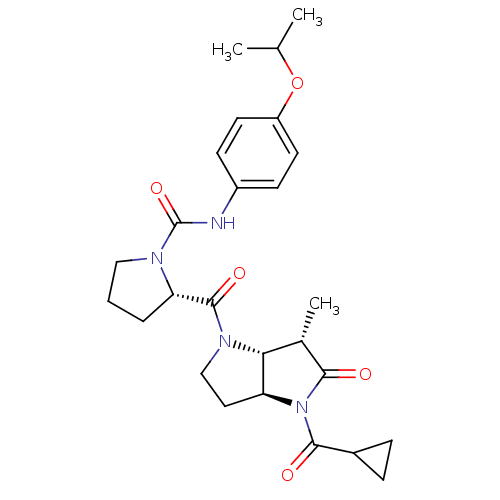 ((2S)-2-{[(3aS,6S,6aR)-4-(Cyclopropylcarbonyl)-6-me...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human herpes virus 5 capsid protein P40 (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133980 ((2S)-2-{[(3aS,6S,6aR)-4-(Cyclopropylcarbonyl)-6-me...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of human cytomegalovirus protease in HCMV pNA assay. | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||