

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50134010 CHEMBL139511::N,N'-(9-(2-aminophenylamino)acridine-3,6-diyl)bis(3-(pyrrolidin-1-yl)propanamide)::N-[9-(2-Amino-phenylamino)-6-(3-pyrrolidin-1-yl-propionylamino)-acridin-3-yl]-3-pyrrolidin-1-yl-propionamide
SMILES: Nc1ccccc1Nc1c2ccc(NC(=O)CCN3CCCC3)cc2nc2cc(NC(=O)CCN3CCCC3)ccc12
InChI Key: InChIKey=NHRMPDKSXMUOQJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50134010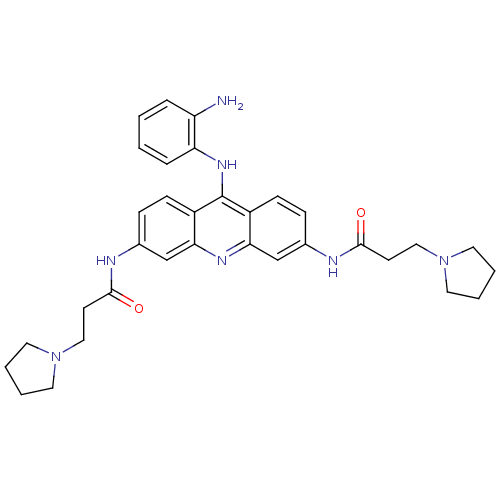 (CHEMBL139511 | N,N'-(9-(2-aminophenylamino)acridin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas Curated by ChEMBL | Assay Description Inhibition of telomerase in human A2780 cells by TRAP assay | Eur J Med Chem 44: 4826-40 (2009) Article DOI: 10.1016/j.ejmech.2009.07.029 BindingDB Entry DOI: 10.7270/Q20R9PHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50134010 (CHEMBL139511 | N,N'-(9-(2-aminophenylamino)acridin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Inhibitory activity against human telomerase | J Med Chem 46: 4463-76 (2003) Article DOI: 10.1021/jm0308693 BindingDB Entry DOI: 10.7270/Q2RR1XN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||