

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50134187 CHEMBL3735506::US9611249, Compound 8
SMILES: COCCN1CCC(CNC(=O)c2n[nH]c3ccc(cc23)-c2cccc(F)c2F)CC1
InChI Key: InChIKey=QCLXNOBXQUWUEW-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 7 (Homo sapiens (Human)) | BDBM50134187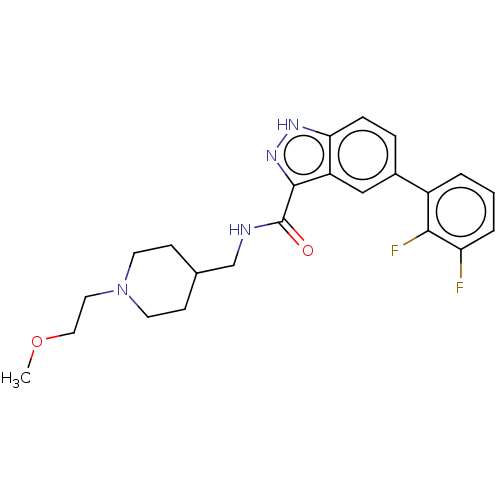 (CHEMBL3735506 | US9611249, Compound 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of human recombinant ERK5 by LANCE assay | J Med Chem 58: 8920-37 (2015) BindingDB Entry DOI: 10.7270/Q2416ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 2 (Homo sapiens (Human)) | BDBM50134187 (CHEMBL3735506 | US9611249, Compound 8) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK2 by LANCE assay | J Med Chem 58: 8920-37 (2015) BindingDB Entry DOI: 10.7270/Q2416ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM50134187 (CHEMBL3735506 | US9611249, Compound 8) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Pharma S.p.A. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHOK1 cells assessed as reduction in tail current by measuring tail current amplitude measured after deploarizat... | ACS Med Chem Lett 11: 825-831 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50134187 (CHEMBL3735506 | US9611249, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AZIENDE CHIMICHE RIUNITE ANGELINI FRANCESCO A.C.R.A.F. S.P.A. US Patent | Assay Description Activity on human GSK-3β was assessed using the following methods (according to Meijer et al., Chem. Biol., 2003-10:1255-1266).In a first screen... | US Patent US9611249 (2017) BindingDB Entry DOI: 10.7270/Q2JH3P79 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50134187 (CHEMBL3735506 | US9611249, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Pharma S.p.A. Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged GSK3beta (1 to 420 end residues) expressed in baculovirus expression system using u... | ACS Med Chem Lett 11: 825-831 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50134187 (CHEMBL3735506 | US9611249, Compound 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of human recombinant GRK2 by LANCE assay | J Med Chem 58: 8920-37 (2015) BindingDB Entry DOI: 10.7270/Q2416ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||