

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50135213 CHEMBL3747016
SMILES: Cn1ccc(n1)-c1ccc(Cn2cc(C(=O)N[C@H]3CCOC[C@@H]3O)c3ncccc23)cc1
InChI Key: InChIKey=FQGJDGJXEZTKDW-UNMCSNQZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50135213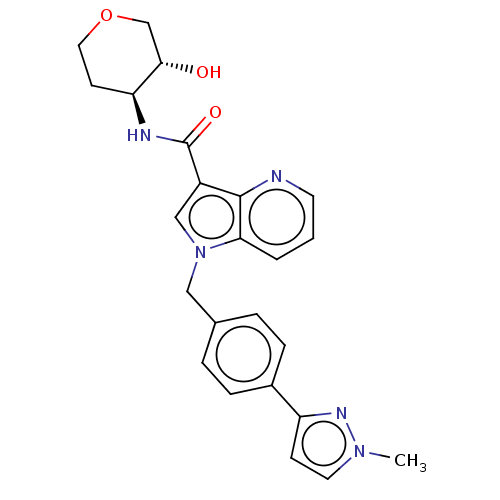 (CHEMBL3747016) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against [3H]- (+)-3-PPP binding to Sigma opioid receptor in guinea pig brain membrane homogenates | J Med Chem 60: 6649-6663 (2017) Article DOI: 10.1021/acs.jmedchem.7b00597 BindingDB Entry DOI: 10.7270/Q2ZK5JS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50135213 (CHEMBL3747016) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Positive allosteric modulation of human muscarinic M1 receptor expressed in CHO cells assessed as increase in ACh-induced intracellular calcium level... | Bioorg Med Chem Lett 28: 2068-2073 (2018) Article DOI: 10.1016/j.bmcl.2018.04.054 BindingDB Entry DOI: 10.7270/Q2TB19HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50135213 (CHEMBL3747016) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human muscarinic acetylcholine receptor M1 expressed in CHO cells assessed as increase in calcium mobilization incubated for 10 m... | J Med Chem 60: 6649-6663 (2017) Article DOI: 10.1021/acs.jmedchem.7b00597 BindingDB Entry DOI: 10.7270/Q2ZK5JS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50135213 (CHEMBL3747016) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Positive allosteric modulation of human M1 receptor expressed in CHO cells assessed as acetylcholine-induced calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 26: 650-5 (2016) BindingDB Entry DOI: 10.7270/Q29C708F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50135213 (CHEMBL3747016) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Positive allosteric modulation of human muscarinic acetylcholine receptor M1 expressed in CHO cells assessed as increase in acetylcholine-induced cal... | J Med Chem 60: 6649-6663 (2017) Article DOI: 10.1021/acs.jmedchem.7b00597 BindingDB Entry DOI: 10.7270/Q2ZK5JS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||