

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50135256 (S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-2-methylpiperidin-1-yl)-3-(2-methyl-1H-indol-4-yloxy)propan-2-ol::(S)-1-[(2S,4R)-4-(4-Methoxy-benzo[b]thiophen-2-yl)-2-methyl-piperidin-1-yl]-3-(2-methyl-1H-indol-4-yloxy)-propan-2-ol::CHEMBL129053
SMILES: COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]c(C)cc23)[C@@H](C)C1
InChI Key: InChIKey=MBTNZOCGXGVNLT-ZCNNSNEGSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135256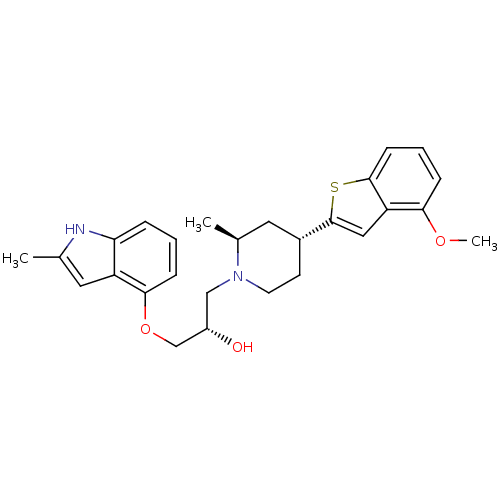 ((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135256 ((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine | Bioorg Med Chem Lett 14: 2653-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.088 BindingDB Entry DOI: 10.7270/Q2GF0SZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135256 ((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50135256 ((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated at 5-hydroxytryptamine 1A receptor labeled with [3H]-8-OH-DPAT | Bioorg Med Chem Lett 14: 2653-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.088 BindingDB Entry DOI: 10.7270/Q2GF0SZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50135256 ((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in human using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50135256 ((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||