

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50135809 CHEMBL150061::MCL-143::di[17-cyclobutylmethyl-(9R,10R)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-trien-4-yl](1R,2S)-cyclopropane-1,2-dicarboxylate
SMILES: O=C(Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1)[C@H]1C[C@H]1C(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1
InChI Key: InChIKey=HFWQBUNFQOLPEO-JBILHQSLSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135809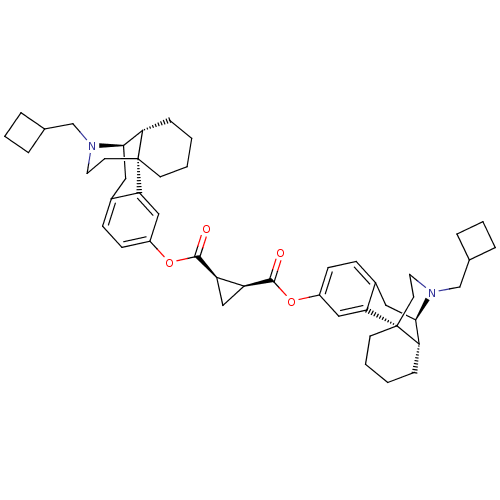 (CHEMBL150061 | MCL-143 | di[17-cyclobutylmethyl-(9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135809 (CHEMBL150061 | MCL-143 | di[17-cyclobutylmethyl-(9...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor mu 1 in chinese Hamster Ovary (CHO) cells membranes was determined using [3H]-DAMGO radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50135809 (CHEMBL150061 | MCL-143 | di[17-cyclobutylmethyl-(9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor delta 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]naltrindole radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||