

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50138040 (biphenyl-3-ylamino)methylenediphosphonic acid::BPH-218::CHEMBL55371::[(Biphenyl-3-ylamino)-phosphono-methyl]-phosphonic acid
SMILES: OP(O)(=O)C(Nc1cccc(c1)-c1ccccc1)P(O)(O)=O
InChI Key: InChIKey=IXFMBXNLOHKCPT-UHFFFAOYSA-N
Data: 14 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cis-Decaprenyl diphosphate synthase (cis-DPPS) (Mycobacterium tuberculosis H37Rv) | BDBM50138040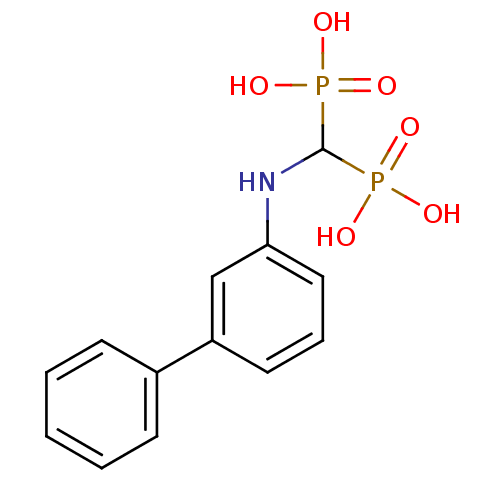 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 53 compounds using trans-FPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by ... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase XIV (Homo sapiens (Human)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl diphosphate synthase (Homo sapiens (Human)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [14C]IPP and GPP as substrate incubated for 10 m... | J Med Chem 55: 3201-15 (2012) Article DOI: 10.1021/jm201657x BindingDB Entry DOI: 10.7270/Q29024V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Plasmodium falciparum (isolate 3D7)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against FPPS in Leishmania major | J Med Chem 49: 215-23 (2006) Article DOI: 10.1021/jm0582625 BindingDB Entry DOI: 10.7270/Q2BV7G6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase (Trypanosoma cruzi) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi hexokinase | J Med Chem 49: 215-23 (2006) Article DOI: 10.1021/jm0582625 BindingDB Entry DOI: 10.7270/Q2BV7G6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl diphosphate synthase (Homo sapiens (Human)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human FPPS | Bioorg Med Chem 16: 8959-67 (2008) Article DOI: 10.1016/j.bmc.2008.08.047 BindingDB Entry DOI: 10.7270/Q2639PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vacuolar-type proton translocating pyrophosphatase 1 (Trypanosoma brucei) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei soluble vacuolar pyrophosphatase expressed in Escherichia coli | J Med Chem 48: 6128-39 (2005) Article DOI: 10.1021/jm058220g BindingDB Entry DOI: 10.7270/Q2F47PX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (MMP14) (Homo sapiens (Human)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of MMP14 catalytic domain (unknown origin) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate incubated for 30 mins prior to substrate... | Bioorg Med Chem 21: 6456-65 (2013) Article DOI: 10.1016/j.bmc.2013.08.054 BindingDB Entry DOI: 10.7270/Q2222W7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase (Trypanosoma cruzi) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Venezolano de Investigaciones Cient£ficas Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi hexokinase | J Biol Chem 282: 12377-87 (2007) Article DOI: 10.1074/jbc.M607286200 BindingDB Entry DOI: 10.7270/Q2FT8MXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Plasmodium falciparum (isolate 3D7)) | BDBM50138040 ((biphenyl-3-ylamino)methylenediphosphonic acid | B...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||