

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC1=NC(N(C(N)=N1)c1cccc(Cl)c1)c1cccc(Oc2cccc(c2)C(F)(F)F)c1
InChI Key: InChIKey=NQOAVWUDLYOSJF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946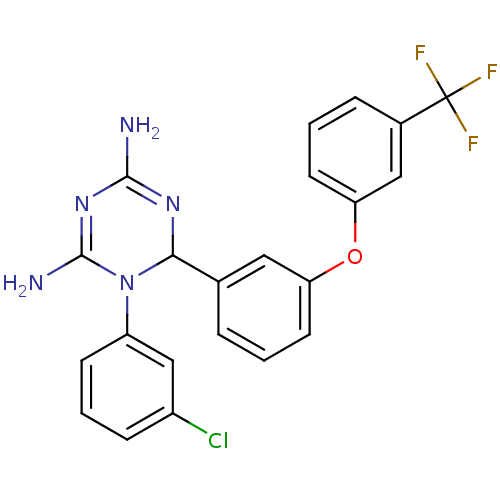 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards mutant dihydrofolate reductase (C59R+S108N+I164L DHFR) of Plasmodium falciparum | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards mutant dihydrofolate reductase (N51I+C59R+S108N DHFR) of Plasmodium falciparum | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards wild-type dihydrofolate reductase of Plasmodium falciparum. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards mutant dihydrofolate reductase (N51I+C59R+S108N+I164L DHFR) of Plasmodium falciparum | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards mutant dihydrofolate reductase (A16V+S108T DHFR) of Plasmodium falciparum. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with mutant A16V+S108T (T9/94RC17) dihydrofolate reductase. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with mutant N51I+C59R+S108N (W2) dihydrofolate reductase. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with wild type dihydrofolate reductase (TM4/8.2). | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with mutant CN51I+C59R+S108N+I164L (V1/S) dihydrofolate reductase. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with mutant C59R+S108N+I164L (Csl-2) dihydrofolate reductase. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138946 (1-(3-Chloro-phenyl)-6-[3-(3-trifluoromethyl-phenox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with mutant C59R+S108N (K1CB1) dihydrofolate reductase. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||