

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50142346 CHEMBL41078::N-[4-(5-Chloro-2-hydroxy-benzyl)-2-oxo-6-trifluoromethyl-1,2-dihydro-quinolin-3-yl]-C,C,C-trifluoro-methanesulfonamide
SMILES: Oc1ccc(Cl)cc1Cc1c(NS(=O)(=O)C(F)(F)F)c(=O)[nH]c2ccc(cc12)C(F)(F)F
InChI Key: InChIKey=IGFJTSGNIPPHTD-UHFFFAOYSA-N
Data: 1 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50142346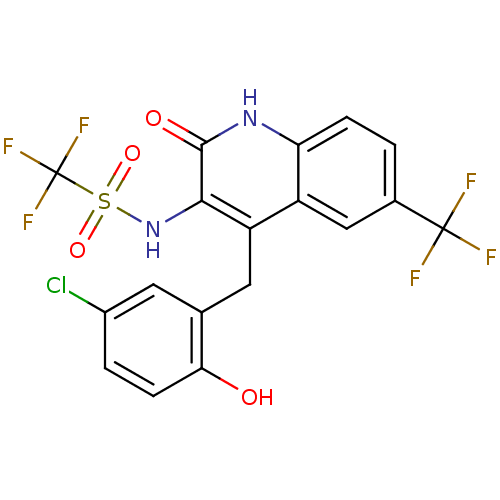 (CHEMBL41078 | N-[4-(5-Chloro-2-hydroxy-benzyl)-2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 663 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Increased outward current at -40 mV mediated by mouse KCNQ2 channel expressed in Xenopus laevis oocytes | Bioorg Med Chem Lett 14: 1615-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.073 BindingDB Entry DOI: 10.7270/Q20V8C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||