

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50142706 CHEMBL3759622
SMILES: CCOC(=O)c1c(CSc2c(Cl)cccc2Cl)[nH]c2c1cc(O)c1ccccc21
InChI Key: InChIKey=DHFZBGQYBOIWIA-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50142706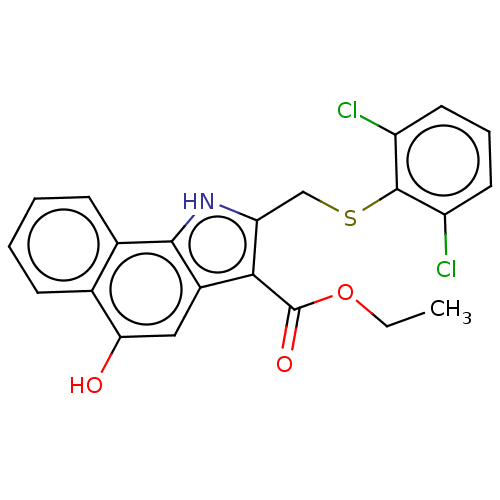 (CHEMBL3759622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of IL-1beta-activated m-PGES-1 in microsome of human A549 cells assessed as PGE2 formation preincubated for 15 mins followed by PGH2 addit... | Eur J Med Chem 108: 466-75 (2016) BindingDB Entry DOI: 10.7270/Q2BR8V14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50142706 (CHEMBL3759622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as LTB4 formation preincubated ... | Eur J Med Chem 108: 466-75 (2016) BindingDB Entry DOI: 10.7270/Q2BR8V14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50142706 (CHEMBL3759622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in human intact polymorphonuclear leukocytes preincubated for 15 mins with CaCl2, A23187 measured after 10 mins by HPLC analysis | Eur J Med Chem 108: 466-75 (2016) BindingDB Entry DOI: 10.7270/Q2BR8V14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||