

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50142796 CHEMBL3759186
SMILES: COc1cc2ncnc(Nc3ccc(Br)cc3)c2cc1OCCCCCCC(=O)NO
InChI Key: InChIKey=BNWIAUUKBJSRSZ-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase (Homo sapiens (Human)) | BDBM50142796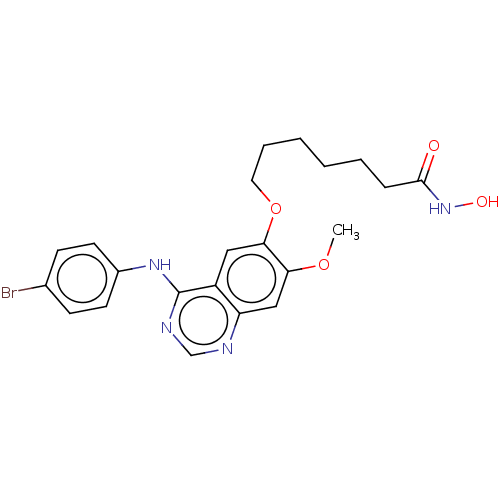 (CHEMBL3759186) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... | Eur J Med Chem 109: 1-12 (2016) BindingDB Entry DOI: 10.7270/Q2377BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50142796 (CHEMBL3759186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) preincubated for 5 mins followed by addition of ATP/gastrin precursor(Tyr87) biotinylated peptide cocktail incu... | Eur J Med Chem 109: 1-12 (2016) BindingDB Entry DOI: 10.7270/Q2377BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50142796 (CHEMBL3759186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay | Eur J Med Chem 109: 1-12 (2016) BindingDB Entry DOI: 10.7270/Q2377BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50142796 (CHEMBL3759186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay | Eur J Med Chem 109: 1-12 (2016) BindingDB Entry DOI: 10.7270/Q2377BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50142796 (CHEMBL3759186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay | Eur J Med Chem 109: 1-12 (2016) BindingDB Entry DOI: 10.7270/Q2377BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50142796 (CHEMBL3759186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay | Eur J Med Chem 109: 1-12 (2016) BindingDB Entry DOI: 10.7270/Q2377BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||