

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50143424 2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid::2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-benzoic acid::2-(6-oxido-3-oxo-3H-xanthen-9-yl)benzoate::3',6'-dihydroxyspiro[1,3-dihydroisobenzofuran-1,9'-(9'H-xanthene)]-3-one::CHEMBL177756::FLUORESCEIN::Fluorescite::Funduscein-25::cid_3383
SMILES: OC(=O)c1ccccc1-c1c2ccc(O)cc2oc2cc(=O)ccc12
InChI Key: InChIKey=YKGGGCXBWXHKIZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solute carrier family 22 member 6 (Mus musculus) | BDBM50143424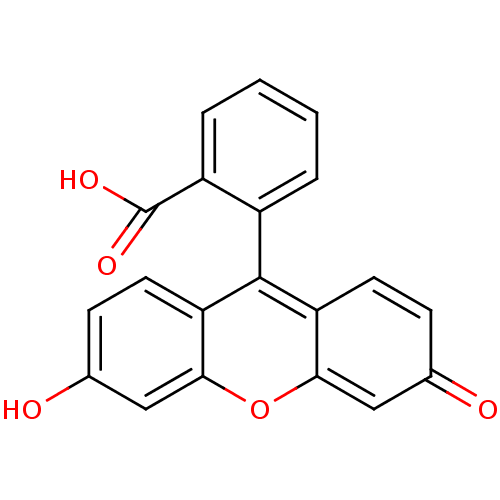 (2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid | ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of mouse Oat1-mediated [3H]PAH uptake in Xenopus oocytes after 1 hr | J Biol Chem 282: 23841-53 (2007) Article DOI: 10.1074/jbc.M703467200 BindingDB Entry DOI: 10.7270/Q2W95B35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 20 (Mus musculus) | BDBM50143424 (2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid | ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of mouse Oat6-mediated [3H]ES uptake in Xenopus oocytes after 1 hr | J Biol Chem 282: 23841-53 (2007) Article DOI: 10.1074/jbc.M703467200 BindingDB Entry DOI: 10.7270/Q2W95B35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50143424 (2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of human PTPase 1B | Bioorg Med Chem Lett 14: 1923-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.079 BindingDB Entry DOI: 10.7270/Q2JS9R01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RmtA (Emericella nidulans) | BDBM50143424 (2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid | ...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of Aspergillus nidulans recombinant GST-RmtA expressed in BL21 cells | J Med Chem 50: 1241-53 (2007) Article DOI: 10.1021/jm061213n BindingDB Entry DOI: 10.7270/Q29887TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1 (Saccharomyces cerevisiae) | BDBM50143424 (2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid | ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibitory activity against Saccharomyces cerevisiae Tyrosine phosphatase 1 | Bioorg Med Chem Lett 14: 1923-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.079 BindingDB Entry DOI: 10.7270/Q2JS9R01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| mothers against decapentaplegic homolog 3 (Homo sapiens (Human)) | BDBM50143424 (2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 4.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: F.M. Hoffmann, University ... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21R6NZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine N-methyltransferase 1 (Homo sapiens (Human)) | BDBM50143424 (2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid | ...) | PDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-PRMT1 expressed in BL21 cells | J Med Chem 50: 1241-53 (2007) Article DOI: 10.1021/jm061213n BindingDB Entry DOI: 10.7270/Q29887TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||