

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50143743 CHEMBL61536::[4-(Benzyl-phenyl-amino)-4'-methyl-[1,4']bipiperidinyl-1'-yl]-(2,4-dimethyl-1-oxy-pyridin-3-yl)-methanone
SMILES: Cc1cc[n+]([O-])c(C)c1C(=O)N1CCC(C)(CC1)N1CCC(CC1)N(Cc1ccccc1)c1ccccc1
InChI Key: InChIKey=YFEPUDFZMYDTMF-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143743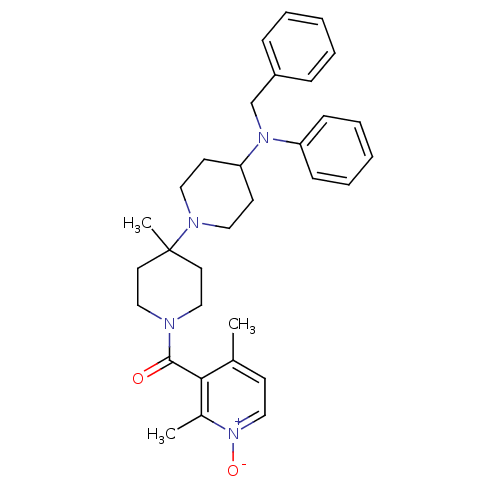 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Migration of compound was evaluated in cell migration assay with C-C chemokine receptor type 5-transfected L1.2 cells or activated human peripheral b... | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143743 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143743 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Rises in intracellular [Ca2+] levels by using [Ca2+] sensitive Fluo4 dye in C-C chemokine receptor type 5-transfected CHO cells. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143743 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 expressed in CHO cells | Bioorg Med Chem Lett 18: 2000-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.108 BindingDB Entry DOI: 10.7270/Q2NC6229 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50143743 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes | Bioorg Med Chem Lett 18: 2000-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.108 BindingDB Entry DOI: 10.7270/Q2NC6229 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50143743 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of radiolabeled MIP-1alpha from cynomolgus monkey CCR5 expressed in CHO cells | Bioorg Med Chem Lett 18: 2000-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.108 BindingDB Entry DOI: 10.7270/Q2NC6229 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143743 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||