

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCOc1cc(C)nc2c(OCc3c(Cl)ccc(N(C)C(=O)CNC(=O)\C=C\c4ccc(cc4)C(=O)NC)c3Cl)cccc12
InChI Key: InChIKey=CCSPRTPOCFHQQR-LFIBNONCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50146893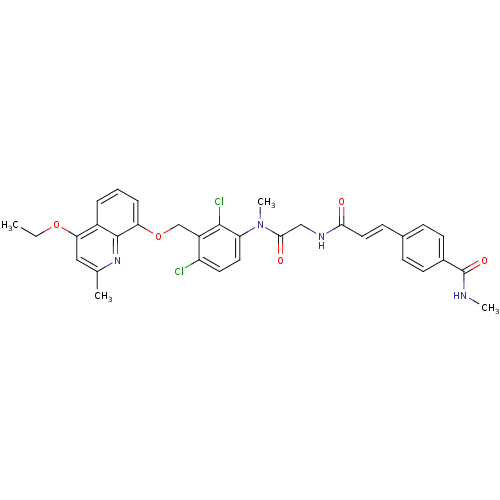 (4-{(E)-2-[({[2,4-Dichloro-3-(4-ethoxy-2-methyl-qui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged KDM4A (1 to 359 residues) expressed in Escherichia coli using biotin-H3K9me3 as substrate preincubated for ... | J Med Chem 59: 810-40 (2016) Article DOI: 10.1021/acs.jmedchem.5b00982 BindingDB Entry DOI: 10.7270/Q2XS5X8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50146893 (4-{(E)-2-[({[2,4-Dichloro-3-(4-ethoxy-2-methyl-qui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit specific binding of [3H]-BK(0.06 nM) to the bradykinin receptor B2 | J Med Chem 47: 2853-63 (2004) Article DOI: 10.1021/jm030468n BindingDB Entry DOI: 10.7270/Q2JM2BCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50146893 (4-{(E)-2-[({[2,4-Dichloro-3-(4-ethoxy-2-methyl-qui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]BK (1.0 nM) binding to the human bradykinin receptor B2, expressed in CHO cells | J Med Chem 47: 2853-63 (2004) Article DOI: 10.1021/jm030468n BindingDB Entry DOI: 10.7270/Q2JM2BCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||