

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50147115 ((1R,2R)-2-Hydroxy-cyclohexyl)-trimethyl-ammonium::CHEMBL102781
SMILES: C[N+](C)(C)[C@@H]1CCCC[C@H]1O
InChI Key: InChIKey=YVFNMIFOQCYHSZ-RKDXNWHRSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Creatine transporter (Rattus norvegicus) | BDBM50147115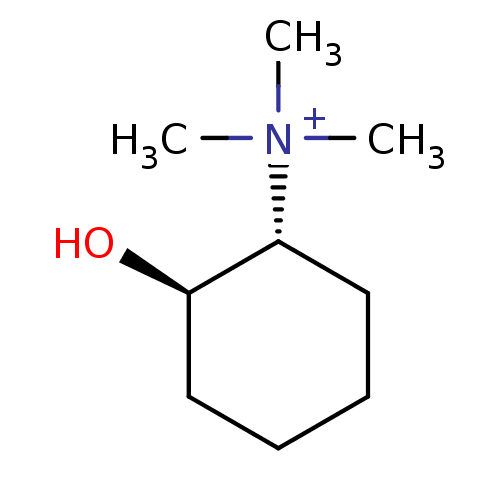 (((1R,2R)-2-Hydroxy-cyclohexyl)-trimethyl-ammonium ...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Tech University Health Sciences Center Curated by ChEMBL | Assay Description Inhibition of [3H]-choline brain uptake was determined by in situ brain perfusion studies in male rats | Bioorg Med Chem Lett 14: 3085-92 (2004) Article DOI: 10.1016/j.bmcl.2004.04.020 BindingDB Entry DOI: 10.7270/Q2BG2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||