

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCOC(=O)N1CCC(CC1)Nc1cc(C)ccn1
InChI Key: InChIKey=LNRMJBWADUSJTA-UHFFFAOYSA-N
PDB links: 3 PDB IDs match this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148162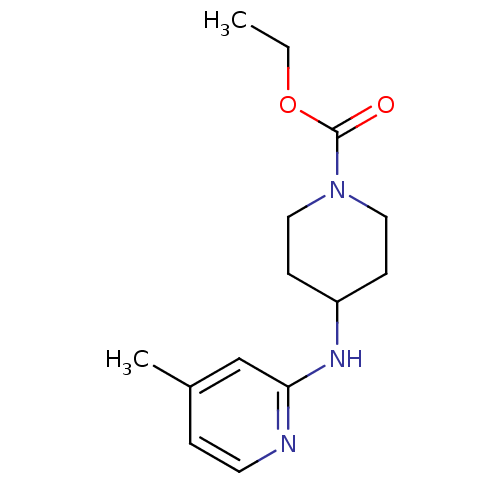 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50148162 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50148162 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50148162 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of wild type human eNOS using L-Arg as substrate incubated for 1 hr prior to L-Arg addition | Eur J Med Chem 58: 117-27 (2012) Article DOI: 10.1016/j.ejmech.2012.10.010 BindingDB Entry DOI: 10.7270/Q29024XX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50148162 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50148162 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148162 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of wild type human iNOS expressed in Escherichia coli BL21(DE3) using L-Arg as substrate incubated for 1 hr prior to L-Arg addition | Eur J Med Chem 58: 117-27 (2012) Article DOI: 10.1016/j.ejmech.2012.10.010 BindingDB Entry DOI: 10.7270/Q29024XX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148162 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||