

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50148763 CHEMBL3142918::[1-[2',5'-Bis-O-(tert-butyldimethylsilyl)-beta-D-ribofuranosyl]-3-N-[3-[[[(hydroxycarbonyl)hydroxyphosphonyl]oxy]-propyl]thymine]-3'-spiro-5''-(4''-amino-1'',2''-oxathiole-2'',2''-dioxide)
SMILES: Cc1cn([C@@H]2O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@]3(OS(=O)(=O)C=C3N)[C@H]2O[Si](C)(C)C(C)(C)C)c(=O)n(CCCOP(O)(=O)C(O)=O)c1=O
InChI Key: InChIKey=LIXVMHIWCAYOGN-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50148763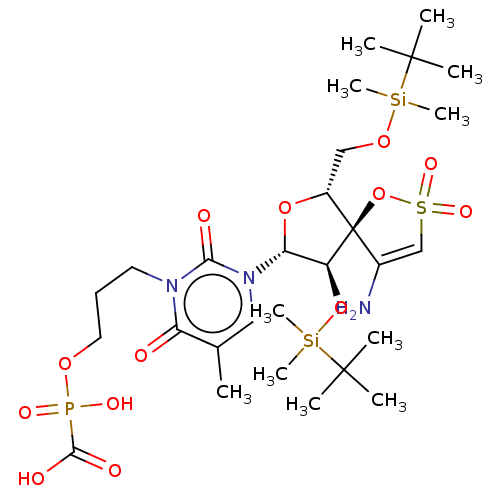 (CHEMBL3142918 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50148763 (CHEMBL3142918 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM, CSIC) Curated by ChEMBL | Assay Description Inhibition of HIV1 3B reverse transcriptase using polyC/oligo(dG12) as template/primer in presence of [3H]dGTP and calf thymus DNA after 30 mins | Eur J Med Chem 150: 206-227 (2018) Article DOI: 10.1016/j.ejmech.2018.03.007 BindingDB Entry DOI: 10.7270/Q2D79F0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50148763 (CHEMBL3142918 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM, CSIC) Curated by ChEMBL | Assay Description Inhibition of TSAO resistant HIV1 3B reverse transcriptase E138K mutant using polyC/oligo(dG12) as template/primer in presence of [3H]dGTP and calf t... | Eur J Med Chem 150: 206-227 (2018) Article DOI: 10.1016/j.ejmech.2018.03.007 BindingDB Entry DOI: 10.7270/Q2D79F0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50148763 (CHEMBL3142918 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||