 Found 8 hits for monomerid = 50149467
Found 8 hits for monomerid = 50149467 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-lactamase (TEM-1)
(Escherichia coli) | BDBM50149467
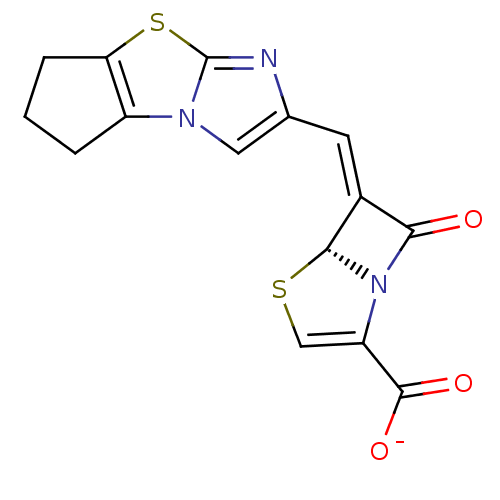
((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Class A (TEM-1) beta-Lactamases |
J Med Chem 47: 3674-88 (2004)
Article DOI: 10.1021/jm049903j
BindingDB Entry DOI: 10.7270/Q2MK6CCT |
More data for this
Ligand-Target Pair | |
Beta-lactamase AmpC
(Escherichia coli) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Class A (Imi-1) beta-Lactamases |
J Med Chem 47: 3674-88 (2004)
Article DOI: 10.1021/jm049903j
BindingDB Entry DOI: 10.7270/Q2MK6CCT |
More data for this
Ligand-Target Pair | |
Beta-lactamase AmpC
(Escherichia coli) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases |
J Med Chem 47: 3674-88 (2004)
Article DOI: 10.1021/jm049903j
BindingDB Entry DOI: 10.7270/Q2MK6CCT |
More data for this
Ligand-Target Pair | |
SHV-1 beta-lactamase
(Klebsiella pneumoniae (Enterobacteria)) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli SHV1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase (TEM-1)
(Escherichia coli) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase type II
(Bacteroides fragilis) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase type II
(Bacteroides fragilis) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Class B (CCRA) beta-Lactamases |
J Med Chem 47: 3674-88 (2004)
Article DOI: 10.1021/jm049903j
BindingDB Entry DOI: 10.7270/Q2MK6CCT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data