

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50150765 (2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)-1-hydroxymethyl-ethylamino]-purin-9-yl}-5-(2-ethyl-2H-tetrazol-5-yl)-tetrahydro-furan-3,4-diol::CHEMBL361925
SMILES: CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccc(Br)cc3)nc12
InChI Key: InChIKey=SSGCBXCKJQWPJL-NIQZGXKPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150765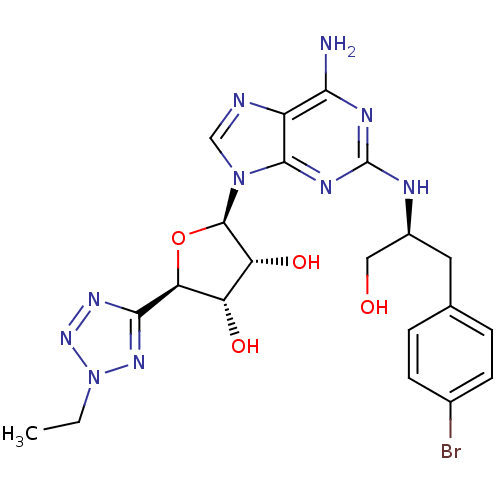 ((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to human adenosine A2A receptor expressed in HeLa cells | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50150765 ((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]NECA binding to human adenosine A3 receptor expressed in HeLa cells | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50150765 ((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]DPCPX binding to human adenosine A1 receptor expressed in CHO cells | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50150765 ((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]DPCPX binding to human adenosine A2B receptor expressed in HEK293 cells | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150765 ((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Potency against cAMP formation in CHO cells expressing recombinant human A2A receptor | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50150765 ((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Potency against cAMP formation in CHO cells expressing recombinant human A2B receptor | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50150765 ((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Relative efficacy against phenylephrine precontracted tissue relaxation in Guinea pig aorta | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine Receptors A2a (A2a) (Rattus norvegicus (rat)) | BDBM50150765 ((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Efficacy against phenylephrine precontracted tissue relaxation in rat aorta | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||