

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50151876 CHEMBL182783::GVNACSSLF
SMILES: CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)CN)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI Key: InChIKey=QRAZOUJGIUABSI-NWHYMTRRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-histidine kinase AgrC (Staphylococcus aureus) | BDBM50151876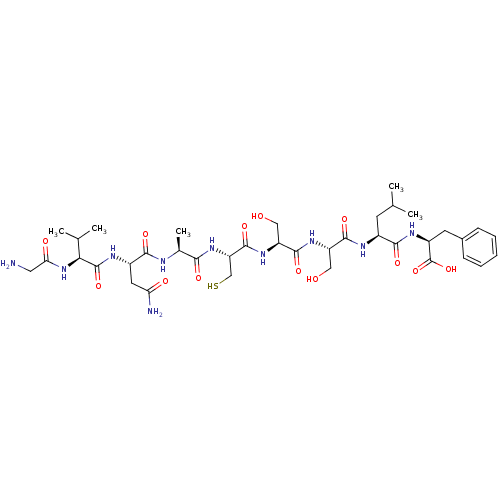 (CHEMBL182783 | GVNACSSLF) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to antagonise accessory gene regulator C4 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-histidine kinase AgrC (Staphylococcus aureus) | BDBM50151876 (CHEMBL182783 | GVNACSSLF) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to antagonise accessory gene regulator C3 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-histidine kinase AgrC (Staphylococcus aureus) | BDBM50151876 (CHEMBL182783 | GVNACSSLF) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Effective concentration to antagonise accessory gene regulator C2 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-histidine kinase AgrC (Staphylococcus aureus) | BDBM50151876 (CHEMBL182783 | GVNACSSLF) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Antagonistic concentration for accessory gene regulator C1 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||