

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50152189 Phosphoric acid 5-(6-amino-purin-9-yl)-2-phosphonooxymethyl-tetrahydro-furan-3-yl ester 4-(6-amino-purin-9-yl)-tetrahydro-furan-2-ylmethyl ester::[4-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl [5-(6-amino-9H-purin-9-yl)-2-[(phosphonatooxy)methyl]oxolan-3-yl] phosphate
SMILES: Nc1ccn(C2COC(COP([O-])(=O)OC3CC(OC3COP([O-])([O-])=O)n3cnc4c(N)ncnc34)C2O)c(=O)n1
InChI Key: InChIKey=LPGFNCONVZCCCG-UHFFFAOYSA-K
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50152189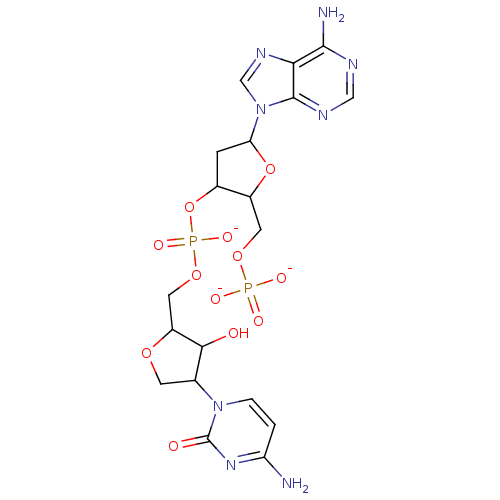 (Phosphoric acid 5-(6-amino-purin-9-yl)-2-phosphono...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibitory activity against 3'-processing step in wild-type HIV-1 integrase was determined using 21-mer oligonucleotide substrate | Bioorg Med Chem Lett 14: 4815-7 (2004) Article DOI: 10.1016/j.bmcl.2004.07.050 BindingDB Entry DOI: 10.7270/Q2QV3N8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50152189 (Phosphoric acid 5-(6-amino-purin-9-yl)-2-phosphono...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibitory activity against strand transfer in wild-type HIV-1 integrase was determined using 21-mer oligonucleotide substrate | Bioorg Med Chem Lett 14: 4815-7 (2004) Article DOI: 10.1016/j.bmcl.2004.07.050 BindingDB Entry DOI: 10.7270/Q2QV3N8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||