

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50153004 CHEMBL3781319
SMILES: COC(=O)Nc1ccc(cc1)-c1ccnc(c1)[C@H](Cc1ccccc1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1
InChI Key: InChIKey=ZBUQQUJRHVSUJI-CWBDRXANSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153004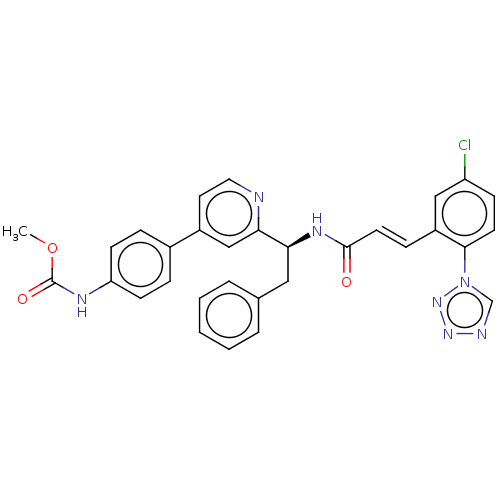 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate measured after 10 to 120 mins by spectrophotometric method | Bioorg Med Chem Lett 28: 987-992 (2018) Article DOI: 10.1016/j.bmcl.2018.02.049 BindingDB Entry DOI: 10.7270/Q2DJ5J7H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analy... | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2/Trypsin-3 (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | >6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation trypsin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human TPA assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | >1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation thrombin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysi... | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | >1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 7a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analys... | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | >2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human urokinase assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | >3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||