

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CS(=O)(=O)c1ccc(cc1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1
InChI Key: InChIKey=VEDMWPYTXDSWTP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153101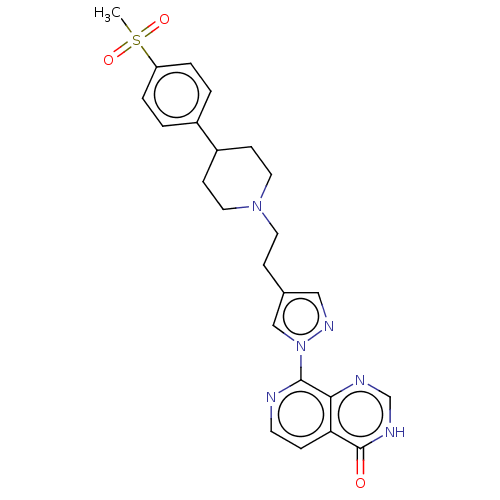 (CHEMBL3774665) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153101 (CHEMBL3774665) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50153101 (CHEMBL3774665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged KDM4A (1 to 359 residues) expressed in Escherichia coli using biotin-H3K9me3 as substrate preincubated for ... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50153101 (CHEMBL3774665) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged KDM4B (1 to 500 residues) expressed in baculovirus infected sf9 cells using biotin-H3K9me3 as s... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||