

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50153344 CHEMBL3774975
SMILES: OC(=O)c1ccncc1NC(=O)c1ccccc1
InChI Key: InChIKey=NBRGBJYDSHESTK-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50153344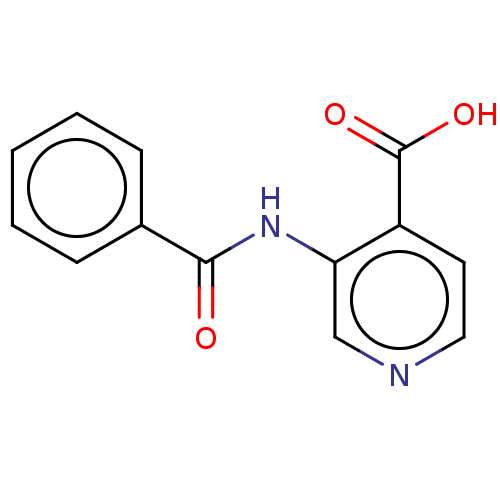 (CHEMBL3774975) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4C (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM50153344 (CHEMBL3774975) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of full length His-MBP-att-EGLN3 (1 to 239 residues) (unknown origin) in Escherichia coli BL21(DE3)pRR692 cells assessed as hydroxylation ... | J Med Chem 59: 1357-69 (2016) BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4D (Homo sapiens (Human)) | BDBM50153344 (CHEMBL3774975) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human KDM4D (11 to 341 residues) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectromet... | J Med Chem 59: 1357-69 (2016) BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 6B (Homo sapiens (Human)) | BDBM50153344 (CHEMBL3774975) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human KDM6B catalytic domain using H3(20 to 36 residues)K27Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire... | J Med Chem 59: 1357-69 (2016) BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||