

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50154041 (S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl-4-phenyl-1H-imidazol-2-yl)piperidin-1-yl)propan-1-one::2-Amino-3-(4-hydroxy-phenyl)-1-[2-(5-methyl-4-phenyl-1H-imidazol-2-yl)-piperidin-1-yl]-propan-1-one::CHEMBL366311
SMILES: Cc1[nH]c(nc1-c1ccccc1)[C@@H]1CCCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1
InChI Key: InChIKey=MKNJLSBSRYKNIQ-SFTDATJTSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50154041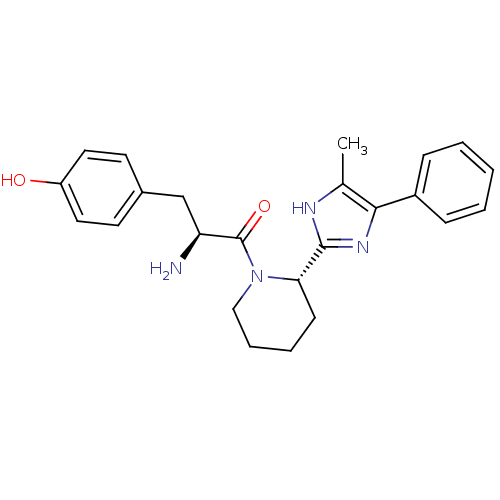 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50154041 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity for Mu opioid receptor of rat brain | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50154041 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor of rat brain | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50154041 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat brain | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50154041 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against delta opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154041 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 142 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50154041 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 542 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154041 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 153 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||