

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50156103 CHEMBL3781521
SMILES: COc1ccc2-c3nn(CCN4CCN(CC4)C(=O)C(C)(C)C)cc3C(C)(C)Oc2c1
InChI Key: InChIKey=PUTMHKMKVABQQZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50156103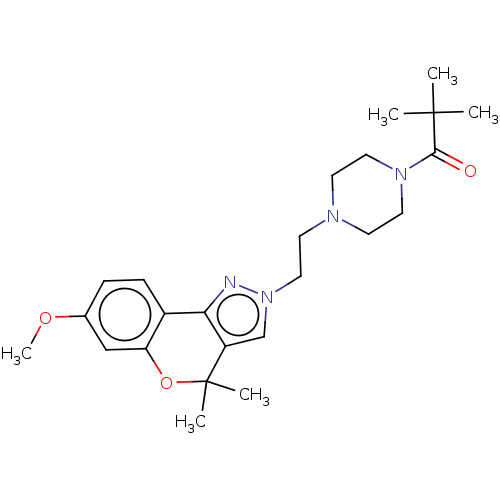 (CHEMBL3781521) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 EBNA cells after 90 mins by liquid scintillation spectrophotometer | J Med Chem 59: 1840-53 (2016) BindingDB Entry DOI: 10.7270/Q2BK1F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50156103 (CHEMBL3781521) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 EBNA cells after 90 mins by liquid scintillation spectrophotometer | J Med Chem 59: 1840-53 (2016) BindingDB Entry DOI: 10.7270/Q2BK1F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM50156103 (CHEMBL3781521) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Antagonist activity at recombinant human GPR55 expressed in HEK293 cells assessed as inhibition of LPI mediated receptor activation by measuring LPI ... | J Med Chem 59: 1840-53 (2016) BindingDB Entry DOI: 10.7270/Q2BK1F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM50156103 (CHEMBL3781521) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Partial agonist activity at recombinant human GPR55 expressed in HEK293 cells after 5 mins by xCELLigence assay | J Med Chem 59: 1840-53 (2016) BindingDB Entry DOI: 10.7270/Q2BK1F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||