

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50156132 CHEMBL3780629
SMILES: COCCNC(=O)c1cc(CCOc2ccc(NC(=O)Nc3cc(nn3-c3ccc(C)cc3)C(C)(C)C)c3ccccc23)ccn1
InChI Key: InChIKey=LYCKQJNDNWHGJD-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 12 (Homo sapiens (Human)) | BDBM50156132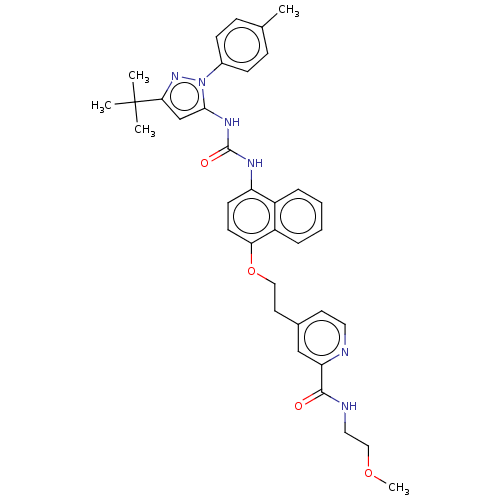 (CHEMBL3780629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sygnature Discovery Limited Curated by ChEMBL | Assay Description Inhibitory concentration required for in vitro binding affinity to cholinergic central Nicotinic acetylcholine receptor on rat brain cortex by using ... | J Med Chem 59: 1727-46 (2016) BindingDB Entry DOI: 10.7270/Q2319XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156132 (CHEMBL3780629) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sygnature Discovery Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant p38 alpha assessed as phosphorylation of MAPKAP-K2 preincubated for 2 hrs followed by FRET peptide and MAPKAP-K2 addi... | J Med Chem 59: 1727-46 (2016) BindingDB Entry DOI: 10.7270/Q2319XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50156132 (CHEMBL3780629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sygnature Discovery Limited Curated by ChEMBL | Assay Description Inhibition of HCK (unknown origin) preincubated for 2 hrs followed by FRET peptide addition measured after 1 hr by Z-LYTE assay | J Med Chem 59: 1727-46 (2016) BindingDB Entry DOI: 10.7270/Q2319XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||