

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50156357 2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol::CHEMBL187158::NN-DNJ
SMILES: CCCCCCCCCN1CC(O)C(O)C(O)C1CO
InChI Key: InChIKey=FTSCEGKYKXESFF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50156357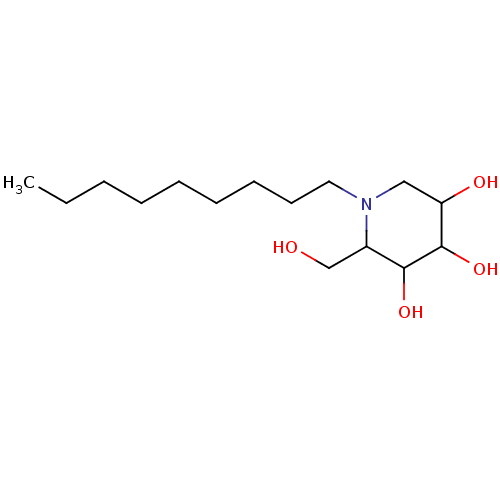 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 488 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 979 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| β-glucosidase (Prunus dulcis (Almond)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.59E+4 | n/a | n/a | n/a | n/a | 10.6 | 37 |
Nankai University | Assay Description In a 96-well plates, 10µL of commercial enzyme solutions without (control) or with 20µL of inhibitor were incubated at 37°C for 5 min.... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| α-glucosidase (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | PDB MMDB Reactome pathway KEGG B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 10.6 | 37 |
Nankai University | Assay Description In a 96-well plates, 10µL of commercial enzyme solutions without (control) or with 20µL of inhibitor were incubated at 37°C for 5 min.... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| α-galactosidase (Coffea arabica (Coffee beans)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.49E+3 | n/a | n/a | n/a | n/a | 10.6 | 37 |
Nankai University | Assay Description In a 96-well plates, 10µL of commercial enzyme solutions without (control) or with 20µL of inhibitor were incubated at 37°C for 5 min.... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Homo sapiens (Human)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | Reactome pathway KEGG GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Prunus avium) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against beta-Glucosidase from sweet almond | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | Reactome pathway KEGG GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against alpha-Glucosidase from rice | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-Glucosidase (α-Glucosidase) (Rattus norvegicus (Rat)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase A (Caldocellum saccharolyticum) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | KEGG GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against beta-Glucosidase from Caldocellum saccharolyticum | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-Glucosidase (α-Glucosidase) (Rattus norvegicus (Rat)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 668 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| alpha-Glucosidase (α-Glucosidase) (Rattus norvegicus (Rat)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||