

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50156457 CHEMBL186730::[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[3-(3-thiophen-2-yl-[1,2,4]oxadiazol-5-yl)-phenyl]-methanone
SMILES: NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cccc(c1)-c1nc(no1)-c1cccs1
InChI Key: InChIKey=YFBRXKLPGVWRRC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156457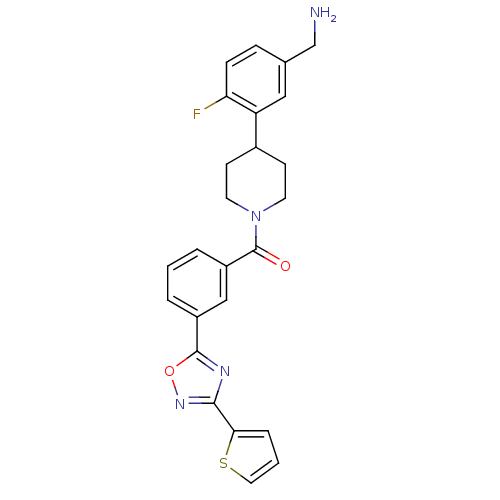 (CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50156457 (CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against cytochrome P450 determined using human liver microsome upon 15 min incubation with probes substrates s-mephenytoin (2C19) | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50156457 (CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Potassium channel HERG | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50156457 (CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against cytochrome P450 determined using human liver microsome upon 15 min incubation with probes substrates tolbutamide (2C9) | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||