

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50157547 2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4-one::7,8,3',4'-tetrahydroxyflavone::CHEMBL222541
SMILES: Oc1ccc(cc1O)-c1cc(=O)c2ccc(O)c(O)c2o1
InChI Key: InChIKey=ARYCMKPCDNHQCL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50157547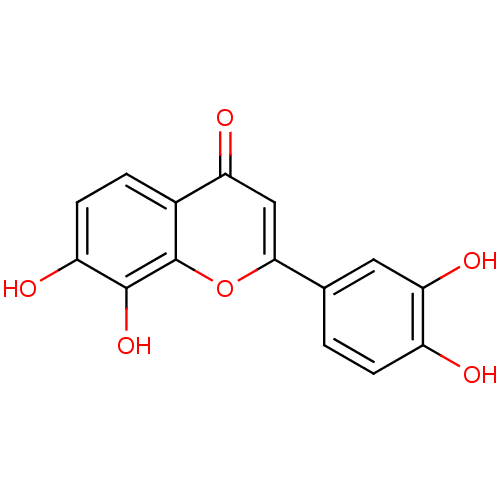 (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
BU-Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human telomerase from HEK293 cell extracts by Flash-Plate assay | J Med Chem 47: 6466-75 (2004) Article DOI: 10.1021/jm040810b BindingDB Entry DOI: 10.7270/Q2P55N1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50157547 (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University Curated by ChEMBL | Assay Description Inhibition of CDK2 | Bioorg Med Chem Lett 17: 1284-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.011 BindingDB Entry DOI: 10.7270/Q2BK1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50157547 (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50157547 (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of telomerase activity (unknown origin) in cell free system using sulforhodamine labeled primer measured after 30 mins by telomerase repea... | Eur J Med Chem 125: 117-129 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50157547 (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of telomerase in human HeLa cells using 5'-AAT CCG TCG AGC AGA GTT-3' as substrate incubated for 15 mins prior to extension reaction follo... | J Med Chem 55: 3678-86 (2012) Article DOI: 10.1021/jm201191d BindingDB Entry DOI: 10.7270/Q2DF6SB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50157547 (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of telomerase in human HeLa cells using 5'-AAT CCG TCG AGC AGA GTT-3' as substrate incubated for 15 mins prior to extension reaction by te... | J Med Chem 55: 3678-86 (2012) Article DOI: 10.1021/jm201191d BindingDB Entry DOI: 10.7270/Q2DF6SB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50157547 (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of telomerase in human HEK293 cells by flashplate assay | J Med Chem 55: 3678-86 (2012) Article DOI: 10.1021/jm201191d BindingDB Entry DOI: 10.7270/Q2DF6SB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||