

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50157555 2-(3,4-dihydroxyphenyl)-4H-chromen-4-one::3',4'-dihydroxy flavone::3',4'-dihydroxyflavone::CHEMBL222556
SMILES: Oc1ccc(cc1O)-c1cc(=O)c2ccccc2o1
InChI Key: InChIKey=SRNPMQHYWVKBAV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipoxygenase-1 (Glycine max (soybean)) | BDBM50157555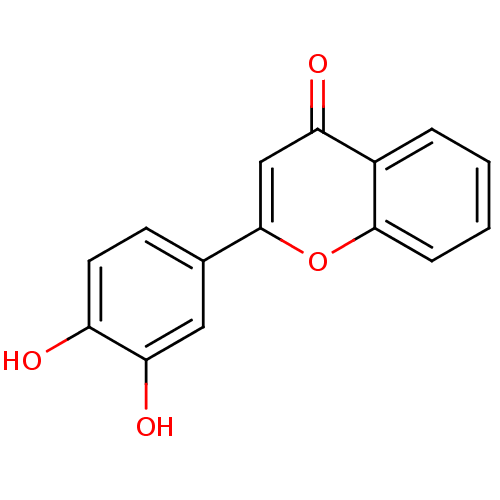 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University Curated by ChEMBL | Assay Description Inhibition of CDK2 | Bioorg Med Chem Lett 17: 1284-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.011 BindingDB Entry DOI: 10.7270/Q2BK1C0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine aminopeptidase (LAP) (Sus scrofa (Pig)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of pig kidney cytosolic Leucyl aminopeptidase | J Nat Prod 58: 823-829 (1995) Article DOI: 10.1021/np50120a001 BindingDB Entry DOI: 10.7270/Q2K937J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Inhibition of 5-LOX-mediated LTB4 production in human neutrophils using arachidonic acid as substrate preincubated for 10 mins followed by substrate ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu Curated by ChEMBL | Assay Description Inhibition of human recombinant ARTD1 by fluorescence assay | J Med Chem 56: 3507-17 (2013) Article DOI: 10.1021/jm3018783 BindingDB Entry DOI: 10.7270/Q24M95W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate multikinase (Homo sapiens) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Inhibition of human IPMK using insP3 as substrate preincubated for 15 mins followed by substrate and measured after 30 mins by TR-FRET assay | J Med Chem 62: 1443-1454 (2019) Article DOI: 10.1021/acs.jmedchem.8b01593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu Curated by ChEMBL | Assay Description Inhibition of human 6XHis-tagged TNKS1 SAM-ART domain (1030 to 1317 amino acid residues) by fluorescence assay | J Med Chem 56: 3507-17 (2013) Article DOI: 10.1021/jm3018783 BindingDB Entry DOI: 10.7270/Q24M95W6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydroorotate dehydrogenase (Homo sapiens (Human)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Bioreactor Engineering Curated by ChEMBL | Assay Description Inhibition of human DHODH expressed in Escherichia coli BL21(DE3) using L-dihydroorotate as substrate after 10 mins by DCIP dye reduction assay | J Med Chem 55: 8341-9 (2012) Article DOI: 10.1021/jm300630p BindingDB Entry DOI: 10.7270/Q2XP762X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate Dehydrogenase (DHODH) (Plasmodium falciparum) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Bioreactor Engineering Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum DHODH expressed in Escherichia coli BL21 (DE3) using L-dihydroorotate as substrate after 10 mins by DCIP dye redu... | J Med Chem 55: 8341-9 (2012) Article DOI: 10.1021/jm300630p BindingDB Entry DOI: 10.7270/Q2XP762X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-chymotrypsin (Bos taurus (bovine)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of alpha-chymotrypsin in bovine pancreas using SPpNA as substrate pretreated with enzyme for 30 mins followed by substrate addition and me... | ACS Med Chem Lett 10: 923-928 (2019) Article DOI: 10.1021/acsmedchemlett.9b00093 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-chymotrypsin (Bos taurus (bovine)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of alpha-chymotrypsin in bovine pancreas using SPpNA as substrate pretreated with enzyme for 30 mins followed by substrate addition and me... | ACS Med Chem Lett 10: 923-928 (2019) Article DOI: 10.1021/acsmedchemlett.9b00093 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol hexakisphosphate kinase 2 (Homo sapiens) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Inhibition of human IP6K2 using insP6 as substrate preincubated for 15 mins followed by substrate and measured after 30 mins by TR-FRET assay | J Med Chem 62: 1443-1454 (2019) Article DOI: 10.1021/acs.jmedchem.8b01593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BU-Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human telomerase from HEK293 cell extracts by Flash-Plate assay | J Med Chem 47: 6466-75 (2004) Article DOI: 10.1021/jm040810b BindingDB Entry DOI: 10.7270/Q2P55N1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu Curated by ChEMBL | Assay Description Inhibition of human 6XHis-tagged TNKS2 ART domain (946 to 1161 amino acid residues) expressed in Escherichia coli Rosetta2 (DE3) by fluorescence assa... | J Med Chem 56: 3507-17 (2013) Article DOI: 10.1021/jm3018783 BindingDB Entry DOI: 10.7270/Q24M95W6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||