

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50157846 3-Hydroxy-7-p-tolyl-1H-furo[3,2-d]pyrimidine-2,4-dione::CHEMBL182381
SMILES: Cc1ccc(cc1)-c1coc2c1[nH]c(=O)n(O)c2=O
InChI Key: InChIKey=FFGCIABZEPVWCP-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA excision repair protein ERCC-5 (Homo sapiens (Human)) | BDBM50157846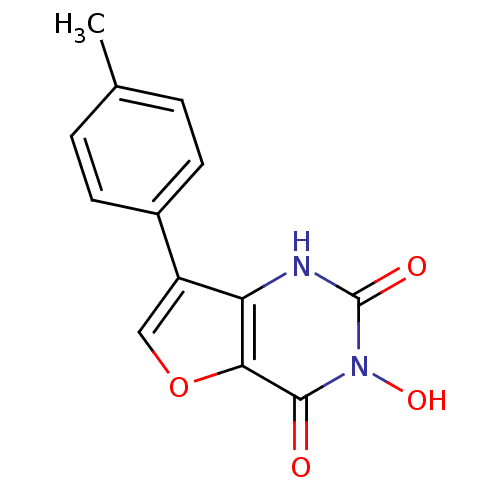 (3-Hydroxy-7-p-tolyl-1H-furo[3,2-d]pyrimidine-2,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a |
Athersys, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against the xeroderma pigmentosum G | Bioorg Med Chem Lett 15: 277-81 (2004) Article DOI: 10.1016/j.bmcl.2004.10.086 BindingDB Entry DOI: 10.7270/Q2HD7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyribonuclease-1 (Homo sapiens (Human)) | BDBM50157846 (3-Hydroxy-7-p-tolyl-1H-furo[3,2-d]pyrimidine-2,4-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology Curated by ChEMBL | Assay Description Inhibition of DNAse1 (unknown origin) | Bioorg Med Chem Lett 25: 4104-8 (2015) BindingDB Entry DOI: 10.7270/Q28S4RQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM50157846 (3-Hydroxy-7-p-tolyl-1H-furo[3,2-d]pyrimidine-2,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology Curated by ChEMBL | Assay Description Inhibition of FEN1 (unknown origin) | Bioorg Med Chem Lett 25: 4104-8 (2015) BindingDB Entry DOI: 10.7270/Q28S4RQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM50157846 (3-Hydroxy-7-p-tolyl-1H-furo[3,2-d]pyrimidine-2,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a |
Athersys, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against the Flap endonuclease-1 | Bioorg Med Chem Lett 15: 277-81 (2004) Article DOI: 10.1016/j.bmcl.2004.10.086 BindingDB Entry DOI: 10.7270/Q2HD7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||