

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50157952 2-Morpholin-4-yl-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-acetamide::2-morpholino-N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide::CHEMBL182634
SMILES: NS(=O)(=O)c1nnc(NC(=O)CN2CCOCC2)s1
InChI Key: InChIKey=CEVFZHVZMHZPRV-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50157952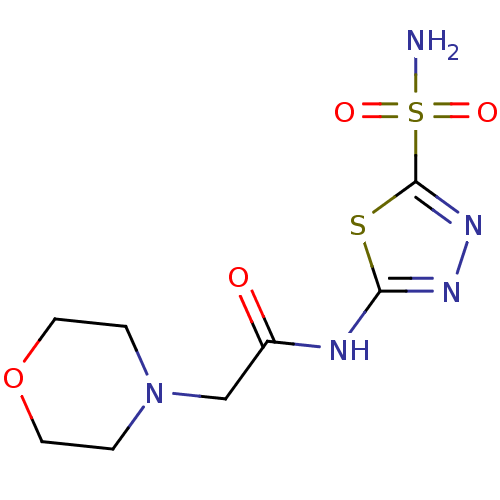 (2-Morpholin-4-yl-N-(5-sulfamoyl-[1,3,4]thiadiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harran University Curated by ChEMBL | Assay Description Inhibitory potency against human cloned Carbonic anhydrase II expressed in Escherichia coli strain BL21 | Bioorg Med Chem Lett 15: 367-72 (2004) Article DOI: 10.1016/j.bmcl.2004.10.070 BindingDB Entry DOI: 10.7270/Q2B858WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50157952 (2-Morpholin-4-yl-N-(5-sulfamoyl-[1,3,4]thiadiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harran University Curated by ChEMBL | Assay Description Inhibitory potency against human cloned Carbonic anhydrase I expressed in Escherichia coli strain BL21 | Bioorg Med Chem Lett 15: 367-72 (2004) Article DOI: 10.1016/j.bmcl.2004.10.070 BindingDB Entry DOI: 10.7270/Q2B858WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50157952 (2-Morpholin-4-yl-N-(5-sulfamoyl-[1,3,4]thiadiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harran University Curated by ChEMBL | Assay Description Inhibitory potency against catalytic domain of human Carbonic anhydrase IX expressed in Escherichia coli strain BL21 | Bioorg Med Chem Lett 15: 367-72 (2004) Article DOI: 10.1016/j.bmcl.2004.10.070 BindingDB Entry DOI: 10.7270/Q2B858WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50157952 (2-Morpholin-4-yl-N-(5-sulfamoyl-[1,3,4]thiadiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harran University Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase 2 assessed as inhibition of carbon dioxide hydration | Eur J Med Chem 44: 3439-44 (2009) Article DOI: 10.1016/j.ejmech.2009.02.016 BindingDB Entry DOI: 10.7270/Q26W9DWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||