

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50158101 CHEMBL3781673
SMILES: NC(=N)c1cccc(c1)N1CC(CN(CC2CCN(Cc3ccccc3)CC2)C1=O)NS(=O)(=O)Cc1ccccc1
InChI Key: InChIKey=GBFNBYMSXRCRBZ-UHFFFAOYSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypsin (Homo sapiens (Human)) | BDBM50158101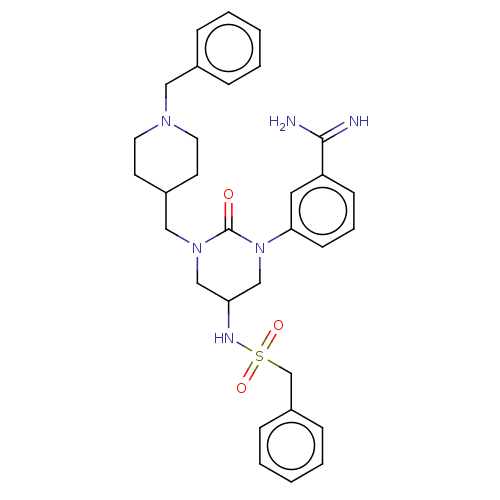 (CHEMBL3781673) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant trypsin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator/Serine protease hepsin/Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50158101 (CHEMBL3781673) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matripase catalytic domain using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158101 (CHEMBL3781673) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepsin (Homo sapiens (Human)) | BDBM50158101 (CHEMBL3781673) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50158101 (CHEMBL3781673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant factor 10a using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50158101 (CHEMBL3781673) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant thrombin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||