

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50158869 CHEMBL3786182::US10836743, Compound GSK-2879552
SMILES: OC(=O)c1ccc(CN2CCC(CN[C@@H]3C[C@H]3c3ccccc3)CC2)cc1
InChI Key: InChIKey=LRULVYSBRWUVGR-FCHUYYIVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869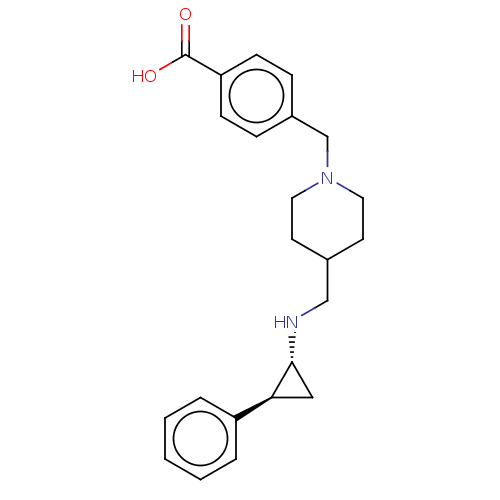 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Time dependant inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system assessed as inhi... | ACS Med Chem Lett 11: 1213-1220 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of KDM1A (unknown origin) by peroxidase coupled assay | J Med Chem 59: 1308-29 (2016) BindingDB Entry DOI: 10.7270/Q2MP5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning Shihua University Curated by ChEMBL | Assay Description Inhibition of recombinant human LSD1 (172 to 852 residues) using biotin-labelled H3K4me2 (1 to 24 residues) after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 29: 844-847 (2019) Article DOI: 10.1016/j.bmcl.2019.01.017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning Shihua University Curated by ChEMBL | Assay Description Inhibition of MAOA (unknown origin) by Promega MAO-Glo assay | Bioorg Med Chem Lett 29: 844-847 (2019) Article DOI: 10.1016/j.bmcl.2019.01.017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning Shihua University Curated by ChEMBL | Assay Description Inhibition of MAOB (unknown origin) by Promega MAO-Glo assay | Bioorg Med Chem Lett 29: 844-847 (2019) Article DOI: 10.1016/j.bmcl.2019.01.017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Screening method: lysine-specific demethylase 1 (LSD1) activity screeningInstrument: microplate reader Envision™ (PerkinElmer, USA).MATERIALS: Human ... | US Patent US10836743 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Screening method: Monoamine oxidase MAOA and MAOB activity screeningInstrument: microplate reader Envision™ (PerkinElmer, USA).MATERIALS: Human recom... | US Patent US10836743 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Screening method: Monoamine oxidase MAOA and MAOB activity screeningInstrument: microplate reader Envision™ (PerkinElmer, USA).MATERIALS: Human recom... | US Patent US10836743 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of LSD1 in human MV4-11 cells assessed as induction of LY96 mRNA expression incubated for 16 hrs by chemiluminescent method | ACS Med Chem Lett 11: 1213-1220 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system using ART-K(Me1)-QTARKSTGGKAPRK... | ACS Med Chem Lett 11: 1213-1220 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Dissociation constant against galectin-3 using competitive fluorescence polarization | Eur J Med Chem 125: 940-951 (2017) Article DOI: 10.1016/j.ejmech.2016.10.021 BindingDB Entry DOI: 10.7270/Q2ZC8597 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Technology of Drug Preparation (Zhengzhou University) Curated by ChEMBL | Assay Description Inhibition of full length recombinant LSD1 (unknown origin) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay | ACS Med Chem Lett 8: 384-389 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869 (CHEMBL3786182 | US10836743, Compound GSK-2879552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of full length recombinant LSD1 (unknown origin) expressed in Escherichia coli BL21(DE3) using H3K4me2 as substrate after 30 mins in prese... | Eur J Med Chem 162: 555-567 (2019) Article DOI: 10.1016/j.ejmech.2018.11.035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||