

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50159163 (3S,4aR,4bS,6R,8R,8aR,10aR)-6-Acetoxy-3-furan-3-yl-4a,8a-dimethyl-1-oxo-dodecahydro-2-oxa-phenanthrene-8-carboxylic acid methyl ester::1-deoxosalvinorin::CHEMBL365533
SMILES: COC(=O)[C@@H]1C[C@@H](C[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1)OC(C)=O
InChI Key: InChIKey=QSBMHYJVPYQAHD-BOPJXXHASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50159163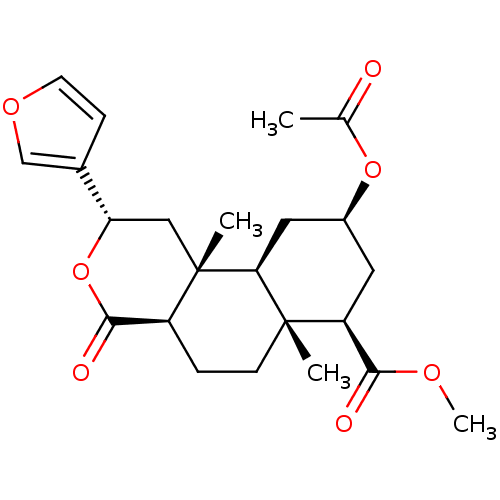 ((3S,4aR,4bS,6R,8R,8aR,10aR)-6-Acetoxy-3-furan-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Melbourne Curated by PDSP Ki Database | Assay Description Inhibition of [3H]-U-69,593 binding to rat opioid receptor kappa 1 expressed in HEK 293 cells | J Med Chem 48: 345-8 (2005) Article DOI: 10.1021/jm049438q BindingDB Entry DOI: 10.7270/Q2CJ8D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159163 ((3S,4aR,4bS,6R,8R,8aR,10aR)-6-Acetoxy-3-furan-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Holden Laboratories Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 6111-5 (2007) Article DOI: 10.1016/j.bmcl.2007.09.050 BindingDB Entry DOI: 10.7270/Q23T9GZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159163 ((3S,4aR,4bS,6R,8R,8aR,10aR)-6-Acetoxy-3-furan-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.34 | n/a | n/a | n/a | n/a |
Holden Laboratories Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor assessed as inhibition of Galpha16-induced calcium flux | Bioorg Med Chem Lett 17: 6111-5 (2007) Article DOI: 10.1016/j.bmcl.2007.09.050 BindingDB Entry DOI: 10.7270/Q23T9GZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||