

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50160555 [(2R,3S,5R)-2-ethynyl-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl]methyl tetrahydrogen triphosphate
SMILES: Cc1cn([C@H]2C[C@H](O)[C@@](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)(O2)C#C)c(=O)[nH]c1=O
InChI Key: InChIKey=DKGZXVAHKCJMQI-YGOYTEALSA-J
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50160555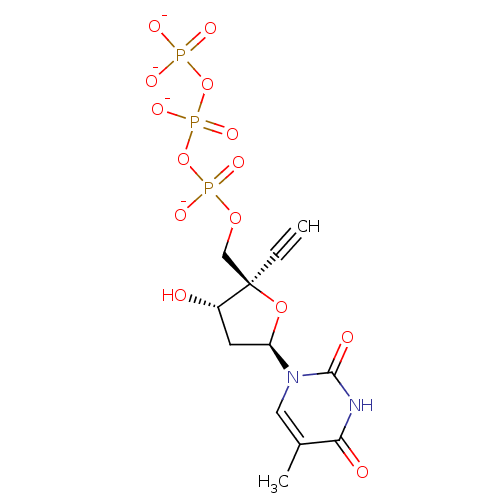 ([(2R,3S,5R)-2-ethynyl-3-hydroxy-5-(5-methyl-2,4-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Konstanz Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1 reverse transcriptase | Bioorg Med Chem Lett 15: 869-71 (2005) Article DOI: 10.1016/j.bmcl.2004.12.072 BindingDB Entry DOI: 10.7270/Q2F190GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||