

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50162108 3-CYCLOHEXYL-1-(2-MORPHOLIN-4-YL-2-OXOETHYL)-2-PHENYL-1H-INDOLE-6-CARBOXYLIC ACID::3-Cyclohexyl-1-(2-morpholin-4-yl-2-oxo-ethyl)-2-phenyl-1H-indole-6-carboxylic acid::3-cyclohexyl-1-(2-morpholino-2-oxoethyl)-2-phenyl-1H-indole-6-carboxylic acid::CHEMBL179532
SMILES: OC(=O)c1ccc2c(C3CCCCC3)c(-c3ccccc3)n(CC(=O)N3CCOCC3)c2c1
InChI Key: InChIKey=ZKEZEXYKYHYIMQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C virus NS5B RNA-dependent RNA polymerase (Hepatitis C virus) | BDBM50162108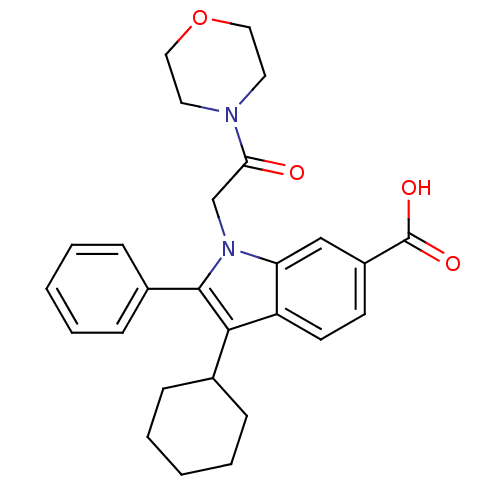 (3-CYCLOHEXYL-1-(2-MORPHOLIN-4-YL-2-OXOETHYL)-2-PHE...) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome) Curated by ChEMBL | Assay Description Effective concentration to inhibit the activity of RNA dependent RNA polymerase nonstructural protein 5B in hepatitis C virus; n=1 | J Med Chem 48: 1314-7 (2005) Article DOI: 10.1021/jm049122i BindingDB Entry DOI: 10.7270/Q2C24VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus NS5B RNA-dependent RNA polymerase (Hepatitis C virus) | BDBM50162108 (3-CYCLOHEXYL-1-(2-MORPHOLIN-4-YL-2-OXOETHYL)-2-PHE...) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase | Bioorg Med Chem Lett 16: 4026-30 (2006) Article DOI: 10.1016/j.bmcl.2006.05.012 BindingDB Entry DOI: 10.7270/Q2416WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus NS5B RNA-dependent RNA polymerase (Hepatitis C virus) | BDBM50162108 (3-CYCLOHEXYL-1-(2-MORPHOLIN-4-YL-2-OXOETHYL)-2-PHE...) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Rome) Curated by ChEMBL | Assay Description Inhibitory activity against RNA dependent RNA polymerase nonstructural protein 5B in hepatitis C virus; n=1 | J Med Chem 48: 1314-7 (2005) Article DOI: 10.1021/jm049122i BindingDB Entry DOI: 10.7270/Q2C24VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||