

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50166450 (R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-amino-2-phenylethyl)-5-(2-fluoro-3-methoxyphenyl)pyrimidine-2,4(1H,3H)-dione::(R)-3-(2-amino-2-phenylethyl)-5-(2-fluoro-3-methoxyphenyl)-1-(2-fluoro-6-(trifluoromethyl)benzyl)pyrimidine-2,4(1H,3H)-dione::3-((R)-2-Amino-2-phenyl-ethyl)-5-(2-fluoro-3-methoxy-phenyl)-1-(2-fluoro-6-trifluoromethyl-benzyl)-1H-pyrimidine-2,4-dione::CHEMBL434936
SMILES: COc1cccc(c1F)-c1cn(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](N)c2ccccc2)c1=O
InChI Key: InChIKey=JZIURJIEJBKSKI-QFIPXVFZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonadotropin releasing hormone 1 (GnRHR1) (Homo sapiens (Human)) | BDBM50166450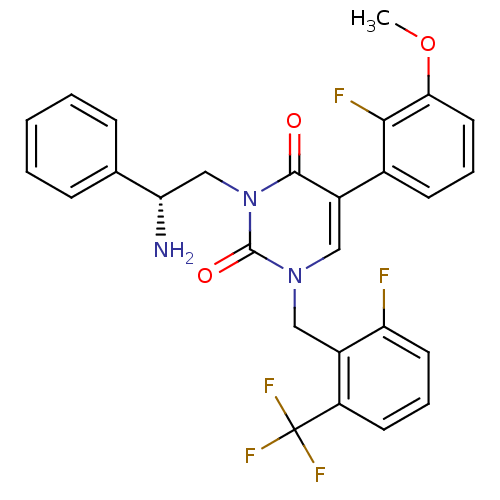 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells | Bioorg Med Chem Lett 18: 4503-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.059 BindingDB Entry DOI: 10.7270/Q2QR4WZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin releasing hormone 1 (GnRHR1) (Homo sapiens (Human)) | BDBM50166450 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to human GnRHR | Bioorg Med Chem Lett 18: 3301-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.036 BindingDB Entry DOI: 10.7270/Q2JD4WKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin releasing hormone 1 (GnRHR1) (Homo sapiens (Human)) | BDBM50166450 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin-releasing hormone receptor (GNRHR) using GnRH peptide as radioligand | Bioorg Med Chem Lett 15: 2519-22 (2005) Article DOI: 10.1016/j.bmcl.2005.03.057 BindingDB Entry DOI: 10.7270/Q2M044ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin releasing hormone 1 (GnRHR1) (Homo sapiens (Human)) | BDBM50166450 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity at human GnRH receptor | J Med Chem 51: 3331-48 (2008) Article DOI: 10.1021/jm701249f BindingDB Entry DOI: 10.7270/Q2KD1XQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin releasing hormone 1 (GnRHR1) (Homo sapiens (Human)) | BDBM50166450 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I-Tyr5,DLeu6,NMeLeu7,Pro9-NEt-]GnRH from human GnRH receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 51: 7478-85 (2009) Article DOI: 10.1021/jm8006454 BindingDB Entry DOI: 10.7270/Q2S46RT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50166450 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 3301-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.036 BindingDB Entry DOI: 10.7270/Q2JD4WKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50166450 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 (unknown origin) by microtiter plate-based fluorimetric assay | Bioorg Med Chem Lett 18: 4503-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.059 BindingDB Entry DOI: 10.7270/Q2QR4WZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50166450 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 51: 7478-85 (2009) Article DOI: 10.1021/jm8006454 BindingDB Entry DOI: 10.7270/Q2S46RT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||