

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50166613 2-{4-[(Butane-1-sulfonylamino)-methyl]-phenyl}-N-[(S)-2-((S)-3-hydroxy-pyrrolidin-1-yl)-1-phenyl-ethyl]-N-methyl-acetamide::CHEMBL362741
SMILES: CCCCS(=O)(=O)NCc1ccc(CC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1
InChI Key: InChIKey=QYAKOPWJZZFMIO-LOSJGSFVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50166613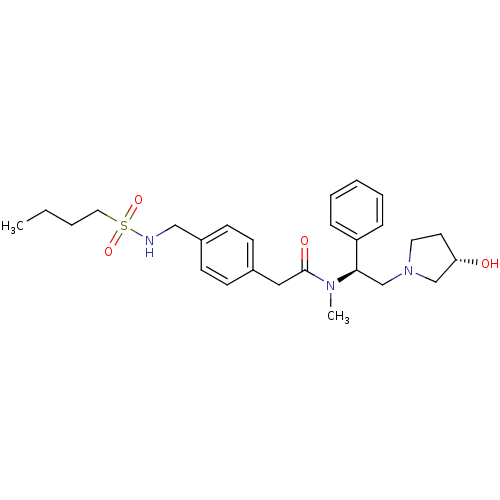 (2-{4-[(Butane-1-sulfonylamino)-methyl]-phenyl}-N-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand | Bioorg Med Chem Lett 15: 2647-52 (2005) Article DOI: 10.1016/j.bmcl.2005.03.020 BindingDB Entry DOI: 10.7270/Q2PR7VH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50166613 (2-{4-[(Butane-1-sulfonylamino)-methyl]-phenyl}-N-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Inhibitory constant against human Opioid receptor delta 1 using [3H]diprenorphine as radio ligand | Bioorg Med Chem Lett 15: 2647-52 (2005) Article DOI: 10.1016/j.bmcl.2005.03.020 BindingDB Entry DOI: 10.7270/Q2PR7VH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50166613 (2-{4-[(Butane-1-sulfonylamino)-methyl]-phenyl}-N-[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Inhibitory constant against human Opioid receptor mu 1 using [3H]diprenorphine as radio ligand | Bioorg Med Chem Lett 15: 2647-52 (2005) Article DOI: 10.1016/j.bmcl.2005.03.020 BindingDB Entry DOI: 10.7270/Q2PR7VH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50166613 (2-{4-[(Butane-1-sulfonylamino)-methyl]-phenyl}-N-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Agonist activity assessed by ability to stimulate [35S]GTP gammaS binding to opioid receptor kappa in human membranes | Bioorg Med Chem Lett 15: 2647-52 (2005) Article DOI: 10.1016/j.bmcl.2005.03.020 BindingDB Entry DOI: 10.7270/Q2PR7VH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50166613 (2-{4-[(Butane-1-sulfonylamino)-methyl]-phenyl}-N-[...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 2D6 was determined using MAMC (7-methoxy-4-aminomethyl-coumarin) as substrate | Bioorg Med Chem Lett 15: 2647-52 (2005) Article DOI: 10.1016/j.bmcl.2005.03.020 BindingDB Entry DOI: 10.7270/Q2PR7VH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||