

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50166737 3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thiazol-4-yl]-benzonitrile::CHEMBL193977
SMILES: N#Cc1cccc(c1)-c1nc(Nc2cccnc2)sc1-n1cncn1
InChI Key: InChIKey=ZNFMHXOUXUCSJG-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166737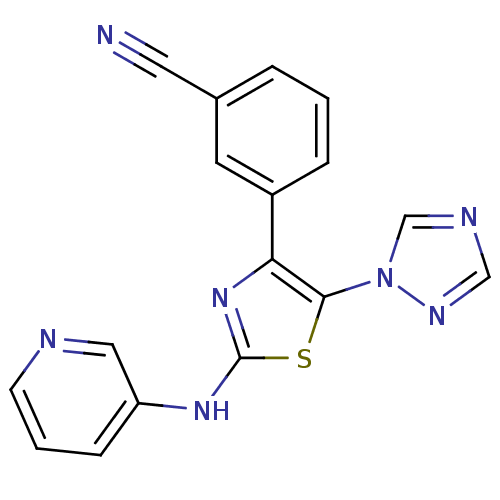 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 698 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||